
Multiyear evaluation of bermudagrass control on creeping bentgrass fairways from herbicides and mechanical slicing
Bermudagrass (Cynodon dactylon L. Pers.) is one of the most troublesome weed species in creeping bentgrass (Agrostis stolonifera L.) throughout the United States transition zone. Aggressive stolon and rhizome development, along with tolerance of bermudagrass
to standard herbicides utilized for weed control in creeping bentgrass, increase the likelihood of bermudagrass invasion. By exploiting winter stress, properly timed physical damage may maximize bermudagrass control. Previous research reported that
topramezone-based herbicide programs have successfully controlled bermudagrass in cool-season turfgrass.
Our preliminary research demonstrated that fall/winter mechanical slicing reduced bermudagrass stand density while having minimal impact on that of creeping bentgrass. So, additional field experiments were conducted at Ballyhack Golf Club, Roanoke, Va.,
from September 2018 to September 2020 to compare topramezone-based herbicide programs alone or combined with mechanical slicing for bermudagrass control in a creeping bentgrass fairway. The experiment was a randomized complete block design with four
replications.
Treatments included a non-treated control, mechanical slicing, topramezone plus triclopyr applied at 0.09 and 0.37 ounces active ingredient per acre (6.4 and 26 grams active ingredient per hectare), respectively, and a combination of mechanical slicing
and topramezone plus triclopyr applied at the same rates. A total of six applications per year of herbicides and slicing were made, with herbicides split equally between fall and spring and slicing initiated in fall and continuing at three-week intervals
into winter. Final bermudagrass cover after two years of herbicide + slicing was 18%, representing 75% control and not different from herbicide without slicing. Bermudagrass area under the cover progress curve per day was 40% over the two-year period
compared to 85% in non-treated turf. Topramezone plus triclopyr programs significantly increased creeping bentgrass coverage and reduced bermudagrass coverage.
Although slicing significantly decreased bermudagrass cover below that of herbicide alone on a few selected evaluation dates, rapid bermudagrass recovery may have reduced its overall effectiveness in these studies.
— Navdeep Godara (ngodara@vt.edu); Shawn D. Askew, Ph.D.; John R. Brewer, Ph.D.; and John N. Peppers, Virginia Tech University, Blacksburg, Va.
Colonization stability and efficacy of plant-growth-promoting rhizobacteria in creeping bentgrass
Inoculation with plant-growth-promoting rhizobacteria (PGPR) is a novel approach to improve growth and abiotic stress tolerance of cool-season turfgrasses. Several endophytic PGPR colonize plant roots and produce ACC deaminase, which reduces the production
of stress-induced ethylene, effectively reducing leaf senescence. However, for this symbiosis to have the intended effects on improving stress tolerance, the roots must first be colonized by the PGPR using successful and confirmed inoculation methods.
Additionally, these methods must be confirmed under fields conditions, which can be challenging due to fluctuating temperatures and moisture, as well as the presence of native soil organisms.
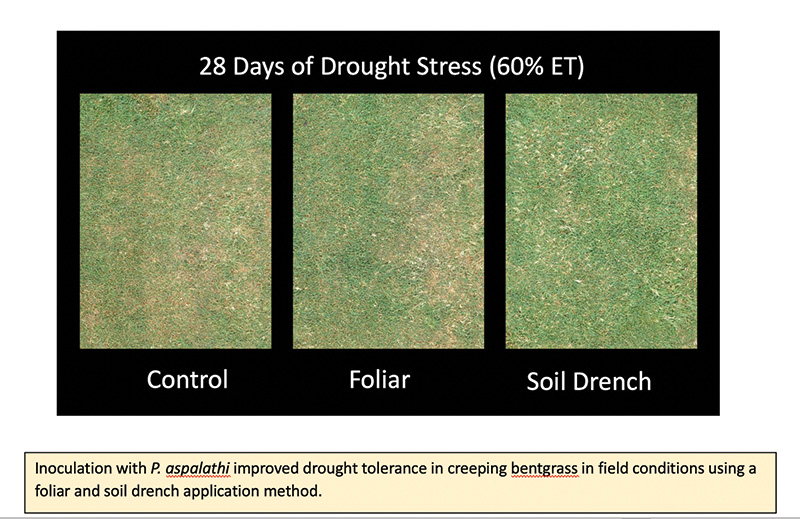
In this study, field plots of creeping bentgrass (Pencross) were inoculated with two novel strains of Paraburkholderia aspalathi bacteria that have demonstrated growth-promoting properties using a foliar spray and soil drench inoculation method. The inoculation
treatments were applied to well-watered plots and plots that were subjected to 28 days of deficit irrigation (60% evapotranspiration) followed by 14 days of rewatering (100% ET). To evaluate the colonization efficiency of bacteria, roots were sampled
from the plots to examine the presence and quantity or density of the bacteria strains that were applied.
The presence of bacterial inoculants in plant tissues was determined by bacterial isolation and quantitative real-time PCR (qPCR) that was designed based on signature sequences of the 16S rDNA in P. aspalathi. Both sequencing analysis and bacterial streaming
from plant tissues were able to confirm that the soil drench method was the most effective inoculation method. Plots inoculated with P. aspalathi using the soil drench method also demonstrated the greatest improvements in drought stress tolerance
and post-drought recovery, suggesting that inoculation with these novel strains of P. aspalathi using the soil drench method is an effective approach to improving drought stress tolerance and reducing water use in creeping bentgrass.
— William Errickson (william.errickson@njaes. rutgers.edu), Kashif Jaleel and Bingru Huang, Ph.D., Rutgers University, New Brunswick, N.J.
Darrell J. Pehr (dpehr@gcsaa.org) is GCM’s science editor.