
Figure 1. Growth of pure cultures of C. jacksonii in response to increasing propiconazole concentrations in potato dextrose agar for A) a DMI-sensitive and B) a DMI-resistant isolate after 48 hours of incubation at room temperature.
Dollar spot is one of the most common diseases that can plague turfgrasses across the world in temperate and subtropical regions. In the North American Great Lakes region, this disease is caused by a fungus called Clarireedia jacksonii (1). Due to the high aesthetic standards of many turf managers, managing this disease often requires frequent fungicide applications. Demethylation inhibitor (DMIs) fungicides, such as propiconazole (the active ingredient in Banner MAXX and Quali-Pro Propiconazole, among others), are a major class of fungicides used today. However, over time, populations of this fungus can become less sensitive to certain families of chemicals, making control more challenging. One example of this is propiconazole, and the Hsiang lab, based out of the University of Guelph, has been monitoring the development of propiconazole resistance in Ontario populations at 10-year intervals since the chemical was registered for use on turfgrass in Canada in 1994 (2, 3, 4, 5, 6). It has found that isolates from sites that had been more intensively treated with DMI fungicides showed a reduced sensitivity to propiconazole compared to the sensitive non-treated (wild-type) populations. Similar resistance issues have even been reported at golf courses in the United States and China (6, 7, 8, 9).
Currently, evaluation of propiconazole resistance requires sending turf samples to a lab for specialized testing, a process that can be slow, costly and inconvenient. To address this, our research team has developed a simple, rapid and cost-effective field kit that allows turf managers to quickly identify propiconazole-resistant strains of C. jacksonii directly from turf samples collected on golf courses. Testing of the kit on over 2,000 symptomatic and over 200 asymptomatic leaf blades from inoculated field research plots showed the kit to be ~96% accurate at identifying propiconazole-resistant isolates. Practical assessment of the field kit was done with the help of participating golf courses and turf managers at 20 locations across southern Ontario. Over 200 leaves displaying symptoms of dollar spot disease were collected by turf managers and assessed using our field kit. We also collected samples at the same time to verify the findings. C. jacksonii was found at 13 of the sampled locations, propiconazole-resistant isolates were found at 12 of these locations, and these results from turf managers’ tests were fully confirmed by our tests with the field kit.
This new tool will allow turf managers to rapidly assess isolates of the dollar spot pathogen for resistance, allowing them to make more informed decisions about fungicide applications, optimize disease control strategies and potentially mediate the development of resistant populations. The current kit includes a few small plates of a growth medium amended with a discriminatory concentration of propiconazole and a set of instructions for identifying the fungus and interpreting the results. We anticipate that this assay can serve as a proof of concept for the development of similar kits for other turfgrass pathogens and even pathogens on other plant hosts.

Figure 2. Symptoms of dollar spot disease on creeping bentgrass at the Guelph Turfgrass Institute: tan-colored spots (0.79-to-1.97-inch diameter/2-to-5-centimeter diameter).
Methods and materials
Fungal growth media
The following growth media were used in the course of this study (1 part per million = 1 microgram per milliliter):
Culturing media: for revival of fungal isolates and the detection of C. jacksonii from field samples. Potato dextrose agar (PDA) was amended with the antibiotics tetracycline hydrochloride (40 ppm) and streptomycin sulfate (100 ppm).
Sensitivity assessment media: for sensitivity differentiation of pure isolates in the lab. Propiconazole (Banner MAXX, Syngenta, Basel, Switzerland) was diluted and added to molten PDA to obtain concentrations of 0.01, 0.1, 1.0 and 10.0 ppm.
Field kit media: for assessment of propiconazole resistant isolates from infected leaf blades. This is the medium that is used in the final kit: PDA amended with propiconazole at 0.5 ppm, tetracycline (40 ppm), streptomycin (100 ppm) and tartaric acid (0.1%).

Symptoms of dollar spot disease on creeping bentgrass at the Guelph Turfgrass Institute: “Cobwebbing” observed with high humidity or dew.
Differentiating between resistant and sensitive isolates
To differentiate between propiconazole-resistant and -sensitive isolates of the dollar spot pathogen, we assessed the growth of 21 isolates, with known sensitivities to DMIs based on previous research and using a scale reported in other studies (7). PDA was amended with propiconazole to concentrations of 0.0, 0.01, 0.1, 1.0 and 10 ppm. By comparing the growth rate across these doses for the selected isolates, we could determine a threshold concentration that significantly reduced (or inhibited) growth of sensitive isolates but still allowed for noticeable growth from resistant isolates (Figure 1). This concentration (0.1 ppm) was then selected as the basis for preliminary field testing of the planned kit.
In-house assessment of the field kit
Initial field tests were conducted at the Guelph Turfgrass Institute (GTI), where two isolates from each of the sensitivity classifications were selected and used to inoculate managed greens. A grid of 24 39.4-inch-by-39.4-inch (1-meter-by-1-meter) plots was established and inoculated with the selected isolates (six isolates and four replications in a randomized complete block design). After inoculation, a one-to-two-week incubation period was used to allow for the fungus to infect and produce symptoms. Once symptoms were observed, symptomatic leaves were collected from each plot twice a week for up to four weeks and assessed using the field kit. To ensure we were sampling the dollar spot fungus and not another pathogen, additional leaves were collected from each treatment plot and incubated on the culturing media. The fungus was then identified based on the rapid growth rate and white cottony hyphal growth that is characteristic of this fungus (2). All plates were incubated at room temperature for up to 96 hours.

Symptoms of dollar spot disease on creeping bentgrass at the Guelph Turfgrass Institute: Hourglass lesion on individual leaf blade.
Practical assessment of the field kit
In the summer of 2022, the field kit was provided to turf managers at participating locations across southern Ontario, in an approximate area of 124 miles by 31 miles (200 kilometers by 50 kilometers), using symptomatic leaves from at least one fairway, tee or green. Each field kit consisted of four 2.36-inch-diameter (6-centimeter-diameter) petri plates containing ~0.27 ounces (~8 milliliters) of medium, four strips of parafilm and a brief set of instructions to assist with C. jacksonii identification (symptoms and fungus) and interpretation of kit results. Leaves displaying symptoms of dollar spot disease were collected and plated at each site by both a turf manager and a researcher. Researcher plates were brought back to the lab and incubated at 71.6 F (22 C), while turf managers were instructed to incubate their plates in an indoor shaded location. After 96 hours, all plates were assessed for the presence of C. jacksonii hyphae, and a sensitivity classification was assigned based on the extent of hyphal growth. If fungal growth extended < 0.04 inch (1 millimeter) out from a leaf blade after 96 hours, the isolate was classified as sensitive to propiconazole. If the growth was > 0.04 inch, it was classified as resistant to propiconazole.
To verify the presence of C. jacksonii at each location, additional symptomatic leaves were collected from each site and transported back to the lab. These leaves were surface-sterilized, placed on PDA and incubated for up to 96 hours. If fungal growth consistent with C. jacksonii hyphae was observed, the presence of the fungus was confirmed.
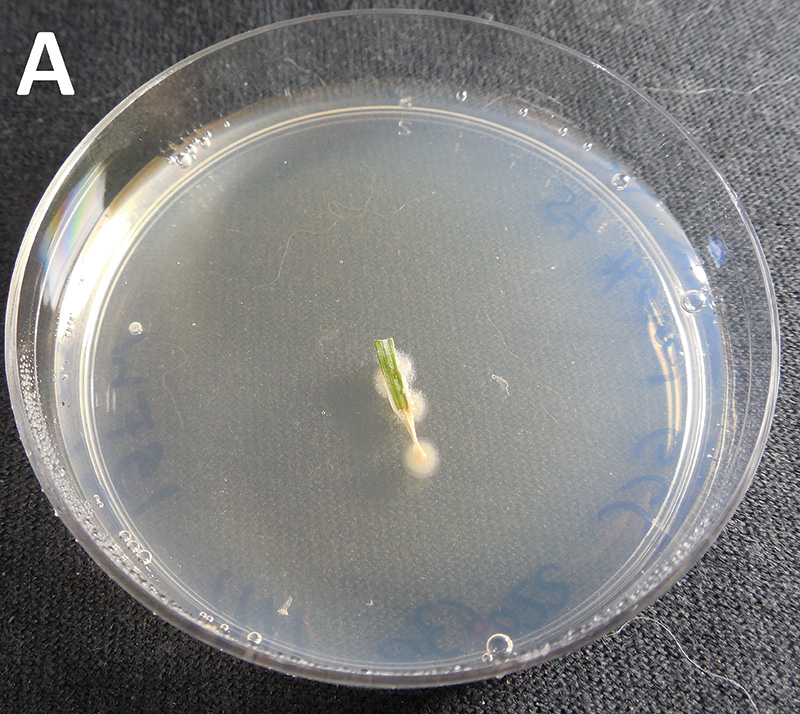
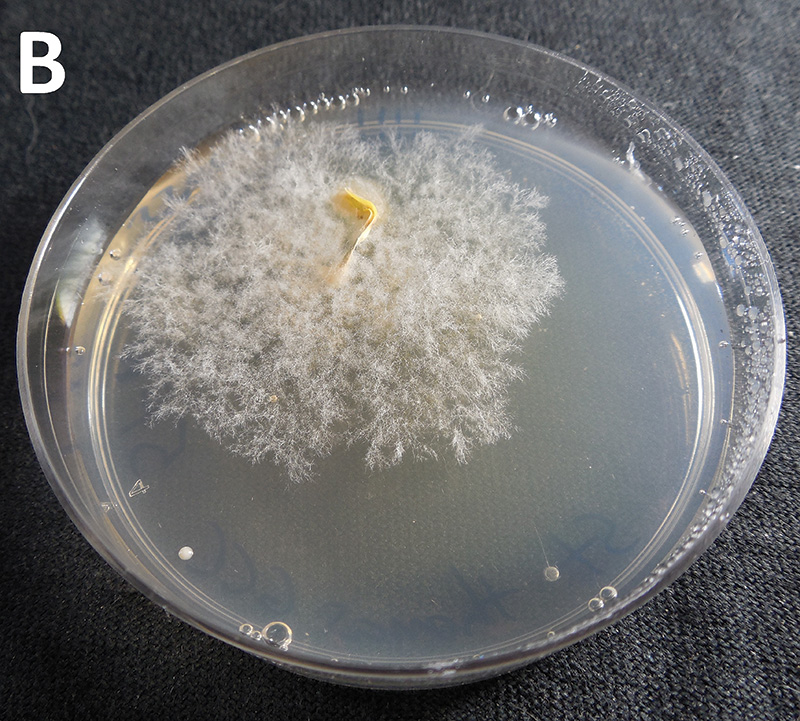
Figure 3. Example of field kit results showing propiconazole (A) sensitive or (B) resistant isolate of C. jacksonii from leaf blades collected from golf courses in southern Ontario after 96 hours.
Results
Twenty-one isolates of C. jacksonii were retrieved from cold storage at the Hsiang lab. The propiconazole sensitivity of each isolate was assessed using media amended with five concentrations of propiconazole (0.0, 0.01, 0.1, 1.0 and 10.0 ppm). Seven of the isolates were classified as sensitive, three as moderately resistant and nine as resistant to propiconazole consistent with previous assessments. Resistant, moderately resistant and sensitive isolates were easily distinguishable by eye at propiconazole concentrations above 0.01 ppm. A concentration of 1.0 ppm was selected for in-house field trials, as sensitive isolates were completely inhibited at this concentration.
During our in-house field trials, five research plots were established at the GTI in summer 2021 and summer 2022. For the first trial, the mean hyphal growth from over 200 symptomatic leaves was assessed using PDA amended with 1.0 ppm propiconazole. But leaves inoculated with a resistant isolate produced only 0.11 ± 0.016 inch (2.8 ± 0.4 millimeters) of hyphal growth after 96 hours, significantly less than the growth observed during the initial lab tests. Additionally, no hyphal growth was observed from leaves inoculated with sensitive or moderately resistant isolates when using the medium, and approximately 10% of the leaves placed on the media were obscured by a contaminating fungus.
In response to the reduced hyphal growth and high bacterial contamination, the amount of propiconazole in the media was reduced to 0.5 ppm to allow for more hyphal growth, especially from moderately resistant isolates, and the antibiotics tetracycline and streptomycin were added to inhibit bacterial growth. Tartaric acid (0.1%) was added to reduce the growth of fast-growing bacteria and fungi. This was the final medium in the field kit, which was tested in-house and by turf managers. Across the second to fifth in-house field trials, more than 600 leaves were collected and assessed using the final medium, which after 96 hours of incubation resulted in increased hyphal growth for propiconazole-resistant (0.39 ± 0.006 inch/10.0 ± 0.15 millimeters) and moderately resistant (0.09 ± 0.02 inch/2.2 ± 0.6 millimeters) isolates, but inhibited growth for sensitive isolates (< 0.0004 inch/< 0.01 millimeter). From these results, a minimum growth of 0.2 inch (5 millimeters) after 96 hours of incubation was set as the threshold for an isolate to be classified resistant. Non-target contaminants occurred on 8.2% of sampled leaves incubated on the final medium; however, they did not appear to adversely affect C. jacksonii growth, but in 6.8% of the cases, C. jacksonii hyphae were obscured by rapidly growing contaminants, which were further identified by DNA sequencing. The quantity and identity of the fast-growing contaminants, such as Mucorales in our case, may differ by location and with environmental conditions and ranged from 0% to 10% for individual locations.
For the practical assessment of the field kit, a short set of instructions showing typical dollar spot symptoms (Figure 2), physical characteristics of the fungus, and examples of sensitive and example results (Figure 3) was prepared and bundled with the 2.36-inch-diameter plates for turf manager testing. The instructions provided with the field kit can be found at: https://bit.ly/41TJ9W0, pages 127-128, and detailed instructions on preparing the field kit growth media are on pages 45-46.
More than 100 symptomatic leaves were collected and assessed by researchers and turf managers using the field kit across the 20 participating locations in southern Ontario during the summer of 2022 (Table 1). Among the 20 locations, C. jacksonii was found at 13 locations as confirmed by distinctive growth on non-amended PDA, and resistant isolates were identified from samples taken at 12 of them. The detection of resistant isolates at each location was consistent between researcher- and turf-manager-prepared kits in all but one case. The proportion of resistant isolates found at each location ranged from 0% to 100%. The contamination rate in the field assays was, on average, 6% for turf managers and 9% for researchers. The two most common contaminants were identified as members of the Mucor genus (10). The primary feedback from the turf managers who tested the kit was that they were hesitant when selecting leaves that might be symptomatic with dollar spot disease and when identifying C. jacksonii hyphae on plates. However, with some assistance, the assay was well received by the turf managers, who had all been eager to assess their propiconazole-resistance situation.
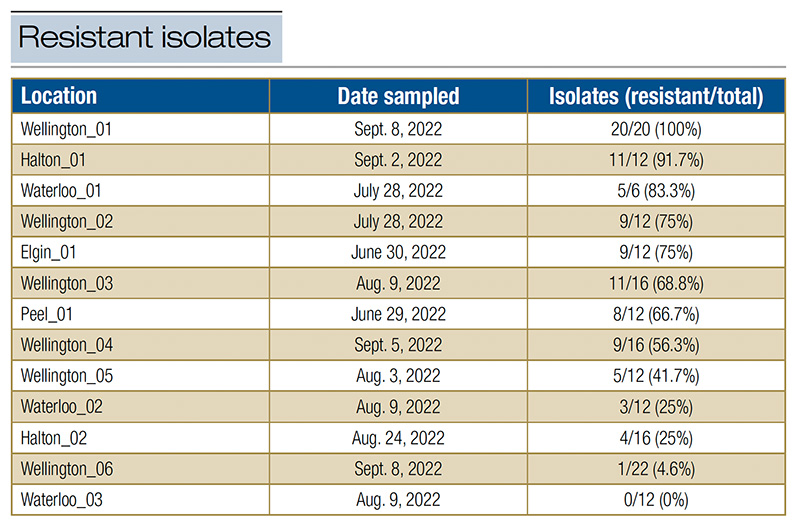
Table 1. Number of propiconazole-resistant isolates of C. jacksonii out of the total number of symptomatic creeping bentgrass leaves assessed in the field assay from each location (specific golf course designated by township) in Ontario during turf manager trials.
Discussion and conclusions
The described field kit was designed with turf managers in mind and to address the growing need for fungicide-resistance monitoring for one of the most common turfgrass pathogens. The assay successfully identified propiconazole-resistant isolates from field samples at 12 locations out of 20 of intensively managed turfgrass in southern Ontario. While we were able to identify resistant isolates at many sampled locations, we were not able to accurately evaluate the intensity of resistance nor the overall proportion of resistant isolates at a given site. To further develop the assay, we are investigating how many samples are needed to confidently assess the level or intensity of resistance at a site and how this may pinpoint the proportion of resistant isolates found (e.g., what percentage of the total isolates being resistant would suggest that there is a potential disease control problem or risk of developing one). Furthermore, as this kit was designed for assessing propiconazole sensitivity, it can be adapted for use with other fungicides with the DMIs as well as other groups.
The final version of our inexpensive kit allowed for quick and easy identification of propiconazole resistant C. jacksonii isolates from field samples, and, when paired with proper instructions, could be used with minimal lab or research experience. This type of assay should be adaptable to other pathogens that are fast growing — > 0.39 inch/day (> 1 centimeter/day) — and easily identifiable in culture so that contaminants pose less of an issue.
The research says
- A discriminatory concentration of propiconazole in a growth medium can be used to identify DMI-sensitive or DMI-resistant isolates of the dollar spot fungus.
- A growth medium containing propiconazole, antibiotics and acids can be used to identify resistant isolates of the dollar spot fungus from field samples.
- An inexpensive field kit based on our selective growth medium can be used by turf managers to identify DMI-resistant isolates of the dollar spot fungus on-site.
Funding
We gratefully acknowledge the support of the Guelph Turfgrass Institute, the Ontario Ministry of Agriculture, Food and Rural Affairs and the Ontario Turfgrass Research Foundation. The original article can be found here: McNab, E., & Hsiang, T. 2024. A practical field assay to detect demethylation inhibitor fungicide resistance in Clarireedia jacksonii. International Turfgrass Society Research Journal (https://doi.org/10.1002/its2.173).
Literature cited
- Salgado-Salazar, C., L.A. Beirn, A. Ismaiel, M.J. Boehm, I. Carbone, A.I. Putman, L.P. Tredway, B.B. Clarke and J.A. Crouch. 2018. Clarireedia: A new fungal genus comprising four pathogenic species responsible for dollar spot disease of turfgrass. Fungal Biology 122(8):761-773 (https://doi.org/10.1016/j.funbio.2018.04.004).
- Hsiang, T., L. Yang and W. Barton. 1997. Baseline sensitivity and cross-resistance to demethylation-inhibiting fungicides in Ontario isolates of Sclerotinia homoeocarpa. European Journal of Plant Pathology 103:409-416 (https://doi.org/10.1023/A:1008671321231).
- Hsiang, T., A. Liao and D. Benedetto. 2007. Sensitivity of Sclerotinia homoeocarpa to demethylation-inhibiting fungicides in Ontario, Canada, after a decade of use. Plant Pathology 56(3):505-507 (https://doi.org/10.1111/j.1365-3059.2007.01573.x).
- Van Den Nieuwelaar, A.M., and T. Hsiang. 2015. Changes in sensitivity of the dollar spot fungus, Sclerotinia homoeocarpa, to the demethylation inhibitor fungicide propiconazole 20 years after first use. European Journal of Turfgrass Science 45:43-44.
- Rether, A., K. Yu and T. Hsiang. 2024. Cross-resistance of Clarireedia jacksonii to demethylation-inhibiting fungicides. International Turfgrass Society Research Journal: In press.
- Golembiewski, R.C., J.M. Vargas, A.L. Jones and A.R. Detweiler. 1995. Detection of demethylation inhibitor (DMI) resistance in Sclerotia homoeocarpa populations. Plant Disease 79:491-493.
- Jo, Y.K., A.L. Niver, J.W. Rimelspach and M.J. Boehm. 2006. Fungicide sensitivity of Sclerotinia homoeocarpa from golf courses in Ohio. Plant Disease 90:807-813 (https://doi.org/10.1094/PD-90-0807).
- Sang, H., J.T. Popko and G. Jung. 2019. Evaluation of a Sclerotinia homoeocarpa population with multiple fungicide resistance phenotypes under differing selection pressures. Plant Disease 103:685-690 (https://doi.org/10.1094/PDIS-06-18-1080-RE).
- Zhang, H., S. Jiang, Z. Zhao, J. Guan, Y. Dong, J. Hu, K. Lamour, S. Yin and Z. Yang. 2021. Fungicide sensitivity of Clarireedia spp. isolates from golf courses in China. Crop Protection 149:105785. (https://doi.org/10.1016/j.cropro.2021.105785).
- McNab, E., and T. Hsiang. 2023. Naturally occurring propiconazole-tolerant fungal isolates in the phyllosphere of Agrostis stolonifera. Journal of Plant Diseases and Protection 131:1195-1201 (https://doi.org/10.1007/s41348-023-00844-3).
Edward McNab (emcnab@uoguelph.ca) is a Ph.D. candidate at the University of Guelph, Ontario. Tom Hsiang, Ph.D. (thsiang@uoguelph.ca), is a professor in the School of Environmental Sciences at the University of Guelph.