
Figure 1. Details of the bioassay method used to document fall armyworm insecticide susceptibility. Photos by Julia N.D. Campos
Insect pests are one of the major threats to turfgrass, including outbreaks of fall armyworm (FAW). The caterpillar activity is most prevalent in the early morning or late afternoon/early evening, remaining hidden the rest of the time within the thatch/mat layer. Fall armyworm insect management is often preventive, and turf managers adopt calendar-based insecticide applications due to the unpredictable risk of decimating turfgrass in a short time, especially in large areas of golf courses.
The susceptibility of FAW to commercially available insecticides can be variable, and when results are less than expected, golf course superintendents report chemical control failure.
Insect resistance is often implicated when repeated failures of an insecticide occur when applications are made properly and in accordance with label recommendations for the targeted pest species. Florida is a key region for documenting FAW susceptibility to insecticides because the region functions as an overwintering and migratory intersection due to warm winter temperatures. Adults have also been documented throughout the year in Florida, and frequent application of insecticides imposes heightened selection pressure on FAW, potentially leading to insecticide resistance. These resistant populations migrate northward each year, infesting other regions of the U.S.
In addition, two host strains of FAW have been identified and are related to host plant preference (5). These host strains, named “C” strain and “R” strain, can only be identified through molecular techniques. However, each strain has unique characteristics (e.g., feeding, development and reproductive behaviors) that impact FAW caterpillar health and viability. In turfgrass, the assumption is that FAW R-strain has a greater survival rate and shorter development time, resulting in an expected higher pest potential (i.e., more damaging insect that is harder to control). However, further documentation of host strain specificity is necessary in turfgrass.
Therefore, the goal of this study was to provide information about FAW host strains’ predominance and performance of insecticides, with the expectation of using this knowledge to improve insect resistance management programs on turfgrass.
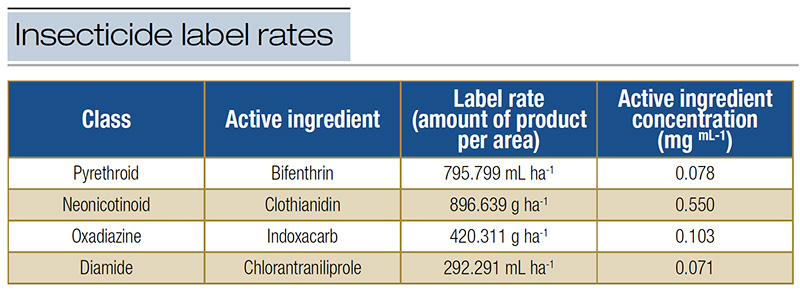
Table 1. Label rate concentration of insecticides commonly adopted for FAW management in turfgrass systems
Material and methods
Field-derived FAW populations
Research was performed in the Entomology Laboratory at the University of Florida, West Florida Research and Education Center (WFREC), Jay, Fla. The FAW moths were collected during the summer and fall in Santa Rosa County, Fla., in the Panhandle. Moth collection used blacklight traps, following Paula-Moraes et al. (6). Light traps were placed along the perimeters of three turfgrass fields in Jay, Roeville and Harold, Fla. The turfgrass system under study included experimental plots in Jay, with a variety of turfgrass cultivars of bermudagrass (Cynodon spp.), zoysiagrass (Zoysia spp.) and St. Augustinegrass (Stenotaphrum secundatum). In Roeville and Harold, the fields were centipedegrass (Eremochloa ophiuroides) grown for sod. The FAW population obtained using the traps is henceforth referred to as the FAW light trap population. A caterpillar collection from FAW was also performed from an outbreak detected in a large experimental area of bermudagrass at WFREC. This population is henceforth referred to as the FAW outbreak population.
The light trap and the outbreak populations were brought to the Entomology Laboratory. The insects were kept in a rearing room at 77 ± 2 F (25 ± 2 C), 70% ± 10% relative humidity, and 12 hour:12 hour light:dark photoperiod. The moths from the F1 generation from both FAW populations were kept and submitted for host strain identification.
Insecticide susceptibility bioassays and strain analysis
Insects from the two FAW populations were subjected to insecticide bioassays using third-instar FAW caterpillars. FAW susceptibility was tested against insecticides from four classes commonly used in turfgrass systems. The insecticides tested included: the pyrethroid bifenthrin (Bifenthrin I/T 7.9F, flowable liquid; ADAMA, Pasadena, Texas), the neonicotinoid clothianidin (Arena, granular; Valent U.S.A. Corporation, Walnut Creek, Calif.), the oxadiazine indoxacarb (Provaunt, granular; Syngenta Crop Protection LLC, Greensboro, N.C.) and the diamide chlorantraniliprole (Acelepryn, suspension concentration; Syngenta Crop Protection LLC, Greensboro, N.C.) (Table 1).
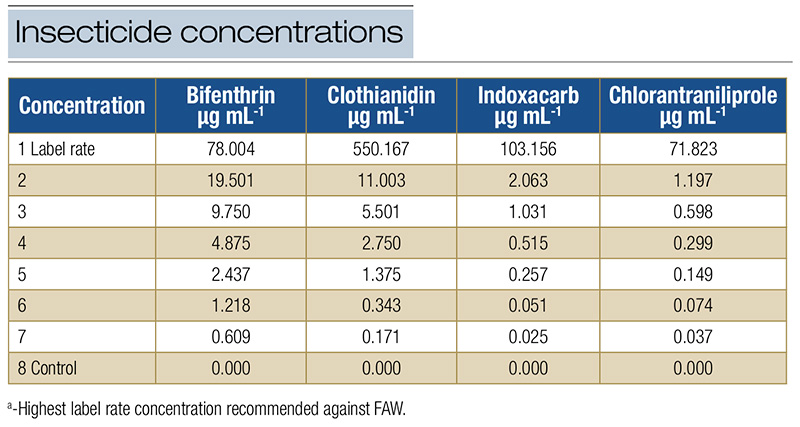
Table 2. Insecticide concentrations tested in bioassays against FAW field-derived populations.
A serial dilution of each insecticide was prepared using distilled water, without adjuvants. Six to seven insecticide concentrations and a control treatment with water were tested (Table 2). The highest concentration used in the serial dilution was the highest labeled rate. The bioassays were conducted using a modified leaf-dip technique (Insecticide Resistance Action Committee — IRAC, Method 007), as described by Campos (2) (Figure 1), which increased plant vigor and longevity, improving the efficacy of insecticide evaluations. The number of replications per concentration varied due to insect availability, ranging from 28 to 33 caterpillars per insecticide concentration. The bioassay evaluations were conducted following IRAC guidelines and in consideration of the mode of action being tested. For bifenthrin, clothianidin and indoxacarb, mortality was assessed 48 hours after exposure, while for chlorantraniliprole, mortality was evaluated 72 hours after exposure. Caterpillars that did not move after being touched with a fine camelhair paintbrush were considered dead. Strain identification was performed using a tissue extraction kit (Qiagen, Germantown, Md.) and genetic markers for DNA comparison (3).
Data analysis
The larval mortality results were analyzed using probit regression in POLO PLUS v1.0. The parameters estimated from the analyses were the median lethal concentration (LC50) and respective confidence intervals (95% CL), the slope of the response curve and standard error (S.E.). The probit model applied to the mortality data of FAW caterpillar feeding on bermudagrass stems treated with chlorantraniliprole, indoxacarb, bifenthrin and clothianidin showed a proper fit (P-value > 0.05) (Table 3). Additionally, chi-square goodness-of-fit tests indicated that observed frequencies in the mortality category matched the model’s expected frequencies, with low chi-square values observed across the light trap and outbreak populations (Table 3).
Results and discussion
The bioassay results demonstrate that the LC50 required to cause mortality to 50% of the FAW population varied among the tested insecticides (Table 3). The FAW light trap population exhibited low LC50 values for indoxacarb and high values for bifenthrin. Similarly, the FAW outbreak population showed LC50 values following the same pattern of susceptibility (Table 3). Fall armyworm populations, when tested against indoxacarb, consistently had the lowest LC50 values, followed by clothianidin, chlorantraniliprole and bifenthrin. These results indicate variable performance of the insecticides commonly used by golf course superintendents to manage FAW, considering the light trap and outbreak populations. However, overall, for all four insecticides, the highest label rate resulted in 100% larval mortality in both FAW light trap and outbreak populations.
The host strain analysis indicated different strain compositions in the two populations (Table 4). Insects from the light trap population had approximately 45% C-strain, 33% R-strain and 22% hybrids. The FAW outbreak population had approximately 3% C-strain, 84% R-strain and 13% hybrids (Table 4).
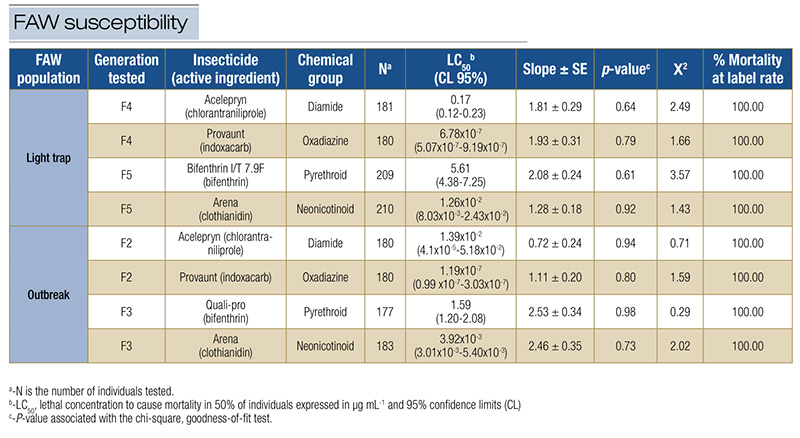
Table 3. Susceptibility of field-derived populations of FAW collected in turfgrass systems and exposed to insecticides in a concentration-response bioassay. Florida Panhandle, Jay, Fla., 2023.
The light trap collection at the WFREC can be considered representative of the FAW population flying across the broader landscape of the Florida Panhandle, which includes urban, forested and agricultural areas. The outbreak population reinforces the predominance of R-strain feeding on turfgrass, one of its preferred host plants. The Florida Panhandle is considered an interbreeding region for FAW populations that overwinter in South Florida and South Texas (1), and light traps may also catch migrating populations from the FAW overwintering sites. In bioassays, using the outbreak population represents an opportunity to generate information and contribute to management strategies against the FAW pest population that was established at the collection site. The results presented here provide information on the current insecticide susceptibility of FAW populations, including dispersal moths caught in traps and caterpillars, across turfgrass landscapes. Currently, the Arthropod Pesticide Resistance Database (APRD) is used as the reference for insecticide baseline of economic pests in the United States and around the world, and up to now, there is no record of FAW insecticide resistance studies in turfgrass.
In addition, this study uncovered notable variations in lethal concentrations (LC50) among tested insecticides and FAW populations. Indoxacarb consistently demonstrated the lowest LC50 values, indicating higher potency against FAW larvae from light trap and outbreak populations. Bifenthrin displayed relatively higher LC50 values, suggesting lower toxicity against FAW under the laboratory bioassay. Our findings suggest that indoxacarb and chlorantraniliprole emerge as the better options for managing FAW among the tested insecticides. However, it is also crucial in insect resistance management programs to consider other factors, such as turf pest diversity and geographical location. In addition, insecticide choice in turfgrass systems is highly context dependent and based on pest monitoring efforts. Bifenthrin may be the most appropriate choice to stop damage in a severe outbreak as a curative application. However, bifenthrin may not be the best option for preventive application in areas with historical recurring infestations since it has a short residual effect. In this scenario, selective insecticides with a more prolonged residual effect, like chlorantraniliprole or indoxacarb, are the better choices. To delay insecticide-resistant populations in high-pressure selection areas, the recommended approach in Insecticide Resistance Management (IRM) programs is to rotate the mode of action and consider information about the specifics of the turfgrass landscape and the timing of FAW infestation.
Moreover, despite variation in susceptibility among tested insecticides, all compounds, at concentrations equivalent to label rates recommended for FAW in turfgrass systems, achieved 100% larval mortality in both populations under study. This study did not account for the residual effect and its impact on FAW insecticide susceptibility. However, the concentration-mortality curves built from each treatment are informative to IRM programs. Considering that the experiment included the best possible scenario (highest label rate) with a uniform insecticide application, the curves also include lower concentrations. In this way, the bioassay replicates situations that can also be observed in the field. The fact that all insecticides are effective against FAW should encourage golf course superintendents to rotate modes of action and diversify management tactics to prevent or delay insecticide resistance. Integrating alternative compounds with distinct modes of action is also the best management practice. In addition, the concentration-response curves estimated in this study provide a quantitative measure, allowing tracking of the evolution of FAW resistance to these insecticides in turfgrass.
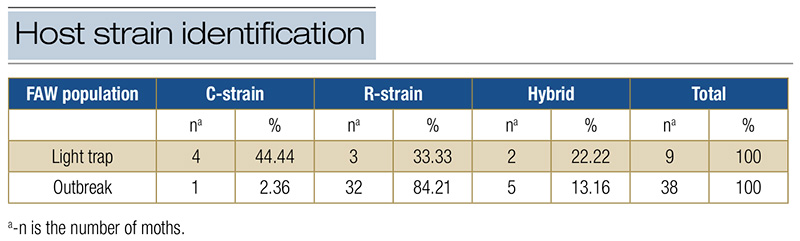
Table 4. Host strain identification using Tpi markers of FAW F1 generation field-derived populations collected in the Florida Panhandle, Jay, Fla., 2023.
The strain identification provides insight into risk assessment. The R-strain is mainly responsible for losses in turfgrass, millet (Cenchrus americanus (L.) Morrone) and other forage grasses, whereas the C-strain is expected to cause most damage to corn (Zea mays L.) and sorghum (Sorghum bicolor (L.) Moench) (4). Knowing the FAW strain may exemplify how different landscapes can be conducive to FAW infestations. Our results confirmed the prediction of R-strain preference and high performance on turfgrass.
Overall, this study provides the first documentation of the performance of insecticides commonly used in Florida turfgrass systems, targeting FAW. Furthermore, the population FAW strain composition is provided, indicating that the R-strain should be targeted in development programs for new insecticide products to manage FAW in turfgrass. Given the migratory behavior of FAW, this study provides valuable insights for other regions cultivating high-input systems with turfgrass. The current efficacy of the four insecticides evaluated suggests that each is suitable for rotation to reduce selection pressure in IRM programs. Continuing FAW susceptibility monitoring studies could be contrasted with the dilution curves developed in this study.
The research says
- The findings suggest that indoxacarb and chlorantraniliprole emerge as the better options for managing FAW among the tested insecticides. However, it is also crucial in insect resistance management programs to consider other factors, such as turf pest diversity and geographical location.
- The fact that all insecticides are effective against FAW should encourage golf course superintendents to rotate modes of action and diversify management tactics to prevent or delay insecticide resistance.
- The current efficacy of the four insecticides evaluated suggests that each is suitable for rotation to reduce selection pressure in insecticide resistance management programs.
Acknowledgments
This research was partially funded by GCSAA.
Literature cited
- Calixto, E.S., and S.V. Paula-Moraes. 2025. Hydrogen stable isotopes indicate reverse migration of fall armyworm in North America. Insects 16(5):471 (https://doi.org/10.3390/insects16050471).
- Campos, J.N.D.G, M.M.P. Rabelo, P.L. Bann, J.B. Unruh, S.V. Paula-Moraes. Insecticide efficacy against tropical sod webworm in concentration-mortality bioassay, 2021. 2022. Arthropod Management Tests 47:tsac009 (https://doi.org/10.1093/amt/tsac009).
- Nagoshi, R.N., J.S. Armstrong, P. Silvie, et al. 2008. Structure and distribution of a strain-biased tandem repeat element in fall armyworm (Lepidoptera: Noctuidae) populations in Florida, Texas and Brazil. Annals of the Entomological Society of America 101(6):1112-1120. (https://doi.org/10.1603/0013-8746-101.6.1112).
- Nagoshi, R.N., and R.L. Meagher. 2022. The Spodoptera frugiperda host strains: What they are and why they matter for understanding and controlling this global agricultural pest. Journal of Economic Entomology 115(6):1729-1743 (https://doi.org/10.1093/jee/toac050).
- Pashley, D.P. 1986. Host-associated genetic differentiation in fall armyworm (Lepidoptera: Noctuidae): A sibling species complex? Annals of the Entomological Society of America 79(6):898-904 (https://doi.org/10.1093/aesa/79.6.898).
- Paula-Moraes, S.V., T.E. Hunt, R.J. Wright, et al. 2012. On-plant movement and feeding of western bean cutworm (Lepidoptera: Noctuidae) early instars on corn. Environmental Entomology 41(6):1494-1500 (https://doi.org/10.1603/EN12149).
Silvana V. Paula-Moraes (silpaulamoraes@gmail.com) is an associate professor in the Department of Entomology at the University of Nebraska-Lincoln. Julia N.D. Campos is a graduate student and J. Bryan Unruh is associate director and professor of turfgrass science at the West Florida Research and Education Center, University of Florida, Jay.