
Figure 1. A sample taken from Hoplolaimus galeatus-infested green at Belmont Golf Course in Richmond, Va.
Creeping bentgrass putting green decline from damage caused by the lance nematode (Hoplolaimus galeatus) is becoming increasingly problematic yet is not well understood. While more attention has been paid to plant parasitic nematodes in recent years, the problem is not new. Increasing anecdotal evidence suggests lance nematodes are part of a larger disease complex associated with environmental and biotic stresses, though limited scientific literature is available. The highest populations of lance nematodes typically coincide with periods of peak environmental stress to creeping bentgrass in August in the northern hemisphere (4). Action thresholds for lance nematodes on golf course putting greens are not well understood, are inconsistent among labs and oftentimes do not coincide with actual reported damage on golf course putting greens. Crow (3) suggested that 200 lance nematodes may cause damage to ultradwarf bermudagrass but also noted no injury in some scenarios with population estimates as high as 1,000 lance nematodes. Damage to creeping bentgrass from lance nematode feeding is speculated to be strongly associated with environmental and other abiotic stress (5, 7).
Figure 1. A sample taken from Hoplolaimus galeatus-infested green at Belmont Golf Course in Richmond, Va.
Nematode samples are commonly sent to diagnostic labs during the spring and summer for assay. Traditional assays require nematode extraction and skillful microscopic identification of individuals, which is time-consuming and often subjective based on expertise. In other areas of turfgrass pathology, molecular methods of identification are commonly used to confirm the presence or absence of a pathogen but have yet to be pursued in great length in identifying lance nematodes (6). A paper by Bae et al. (1) concluded that six Hoplolaimus spp., including H. galeatus commonly associated with turfgrasses, can be amplified using polymerase chain reaction (PCR). These results provide an opportunity for an objective alternative to the routine nematode assay with elutriation and microscopic quantification by using quantitative polymerase chain reaction (qPCR). Quantitative polymerase chain reaction measures amplify DNA in real time by using selected primers and/or probes. The outcome of a qPCR reaction is DNA amplification noted by a cycle threshold (Ct) curve. The lower the Ct value is, the more DNA is present in a reaction. Utilizing qPCR would be new to turfgrass nematode diagnostics and could provide a new diagnostic tool for management decisions.
The objectives of this work were to create and test a qPCR approach for H. galeatus and evaluate the effect H. galeatus may have on creeping bentgrass root biomass as influenced by nitrogen inputs.
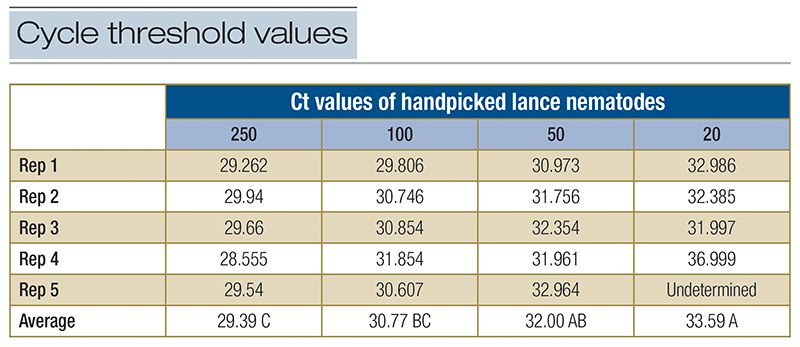
Table 1. Summary table of cycle threshold (Ct) values for handpicked samples of 250, 100, 50 and 20. An analysis of variance (ANOVA) was conducted, and differences are denoted by a different capital letters. Results of undetermined equaled no amplification within a reaction.
Materials and methods
A qPCR approach
A partial sequence of an internal transcribed spacer (ITS) region of H. galeatus was selected from GenBank (EU515322) based on the work completed by Bae et al. (1). Primers were created from this partial sequence using primer3plus with a coverage of 574 base pairs (bp). The forward primer is 20 bp in length containing 50% GC (guanine/cytosine) content, and the reverse primer is also 20 bp in length with 55% GC content. Both forward and reverse primers have secondary hairpin structures. A TaqMan probe was also created for this region of interest to target amplification of H. galeatus and avoid false positives in amplification. This probe has 23 bp, with a 6-FAM reporter on the 5-prime end and an MGBNFQ quencher on the 3-prime end.
Handpicked samples were placed into 10µl of water and micro-pipetted into clean micro centrifuge tubes to a volume of 250µl. Efforts were made with handpicked samples to ensure all nematodes were removed from the original 1.5µl micro centrifuge tube and that no nematodes remained in the micro pipette tip by removing said tip and washing, with a new tip, nematodes that remained into a clean 1.5µl micro centrifuge tube for DNA extraction. A Qiagen Powersoil DNA kit was used to extract DNA from each sample.
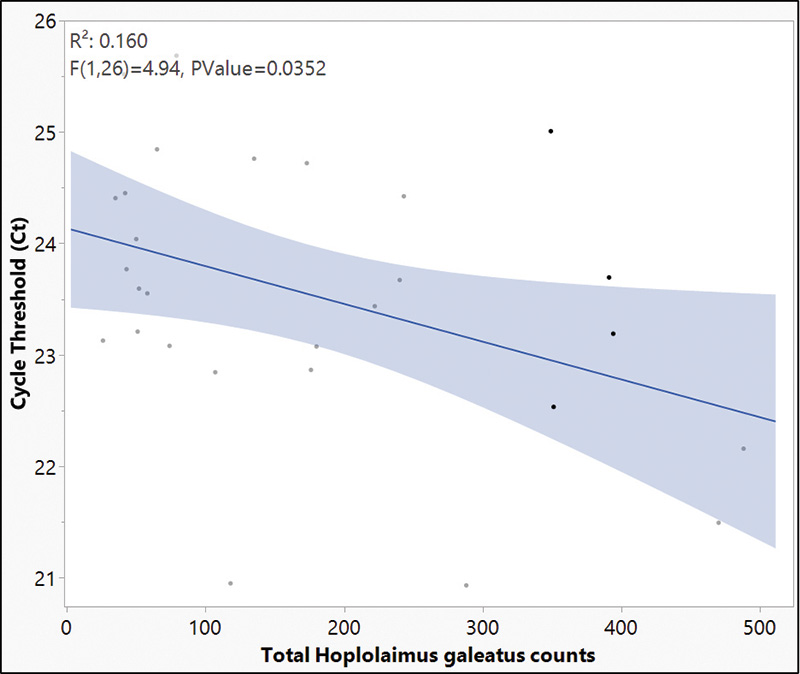
Figure 3. Linear relationship between total lance nematode counts obtained through traditional extraction methods and cycle threshold (Ct) values from samples collected at Belmont Golf Course, Richmond, Va.
qPCR assays were performed using a Thermo Fisher Scientific Quant Studio 3 (Waltham, Mass.). Each assay included 1µl of each primer, 0.5µl of TaqMan probe, 1µl of template DNA, 9µl of distilled water and 12.5µl of SYBR green master mix for a total
reaction volume of 25µl. The qPCR program was modified from Bae et al. (1). Each qPCR run contained a negative control of distilled water in place of the template DNA. Ct values were subject to an analysis of variance with handpicked counts of lance nematodes (ANOVA) in JMP Pro 18 to assess for differences between traditional and molecular methods.
To compare DNA amplification of random samples against traditional nematode counts, samples were collected from Belmont Golf Course in Richmond, Va., from green No. 2 that was previously assessed for presence of H. galeatus (Figure 1). Selection of sample areas from within the green varied based on green size and shape. Twenty-eight sample areas were selected within this green. Greens at Belmont Golf Course are grassed with 777 creeping bentgrass and are grown following USGA recommendations for soil profiles. Nematodes were extracted using a semi-automatic elutriator (2). Samples included the presence of lance, stubby root, ring, stunt, root knot and spiral nematodes. Nematodes were subject to semi-automatic elutriation, centrifugation and sugar flotation. Both a nematode estimation for loss due to elutriation and a total count for lance nematodes were observed and recorded for comparison with qPCR assays. Ct values were compared with total lance nematode counts by linear regression using JMP Pro 18.
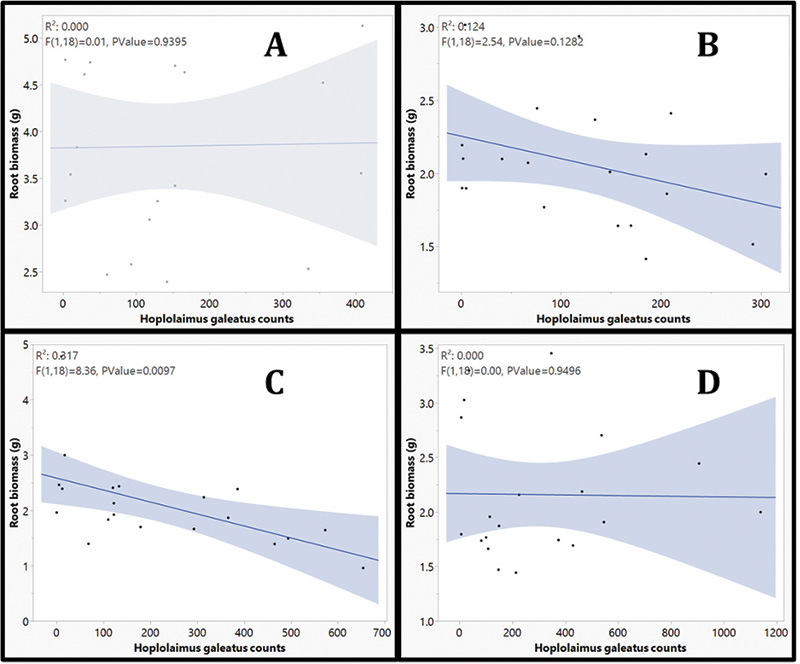
Figure 4. Linear regression of Hoplolaimus galeatus (Cobb, 1913) Thorne, 1935 counts by root biomass of creeping bentgrass (Agrostis stolonifera cv. ‘L93’’) at four destructive sampling dates. Destructive sampling dates were split into four, four-week timings of 4 (A), 8 (B), 12 (C) and 16 (D) weeks after initial urea nitrogen applications.
Evaluate the damage influence of lance nematodes on creeping bentgrass root biomass and compare with plant health spectral reflectance
Two-inch- (5.1-centimeter-) diameter plugs of established 007 creeping bentgrass were removed from a dormant golf course putting green at the Glade Road Research Facility in Blacksburg, Va., and were transplanted into 2-inch Deepot conetainers (Stuewe and Sons Inc., Tangent, Ore.) in the greenhouse. All existing root biomass below the soil/thatch interface was removed by severing with a knife, and the remaining plug was rinsed with water to remove excess soil. Each plug was transplanted to pots prefilled with a sterile 90:10 sand/peat mixture and backfilled with the same mixture as top-dressing. Pots were placed on greenhouse benches, and time was allowed for green-up prior to inoculation and treatment initiation. Plants were inoculated with lance nematodes via soil injection after green-up, and a period of 124 days was allowed for acclamation of nematodes to plants prior to the first nitrogen application (Figure 2). Nitrogen applications were made biweekly using a CO2 backpack sprayer as broadcast applications. Plots were trimmed daily with grooming sheers at a height of 0.125 inch (0.32 centimeter) and watered daily with overhead irrigation to prevent drought stress.
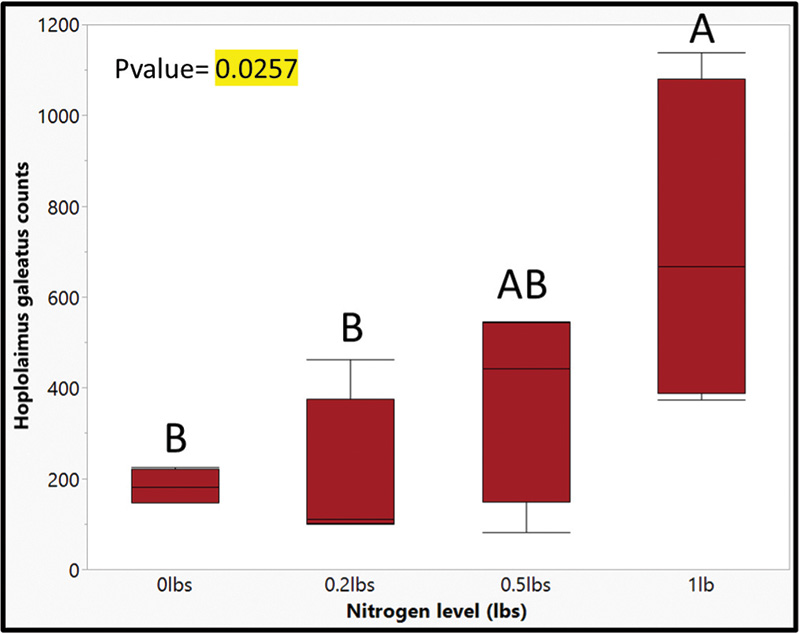
Figure 5. An analysis of variance (ANOVA) for nitrogen level on creeping bentgrass (Agrostis stolonifera cv. ‘L93’’) by Hoplolaimus galeatus (Cobb, 1913) Thorne, 1935 counts 12 weeks after initial nitrogen applications. Bars with the same letter are not statistically significant according to Tukey’s HSD (α=0.05).
A 5x4 factorially arranged design was implemented that involved five population levels of lance nematodes (0, 100, 500, 1,500 and 3,000), and four nitrogen fertilizer rates (0, 0.2, 0.5 and 1 pound/0, 0.09, 0.23 and 0.45 kilogram) were applied over 16 weeks in the form of soluble urea (46% nitrogen-0% phosphorus-0% potassium). A representative of each factor was removed every four weeks and destructively sampled. Destructive sampling included removing nematodes by Baermann trays and a modified Jenkins method followed by collecting dry weights and ashed weights of root biomass present. Root biomass was captured at 4, 8, 12 and 16 weeks by drying root material at 140 F (60 C) for 48 hours then sieving soil from root material using a 60-mesh sieve before measuring dry weights. Dry weights of each sample were subjected to a muffle furnace at 932 F (500 C) for eight hours before being measured for ash weights. Root biomass was determined by subtracting ashed weights from dry weights and recorded in grams. Spectral canopy reflectance was collected daily using a PSR 1100 handheld radiometer with a spectral range of 320-1100 nanometers and a sampling bandwidth of 1.4 nanometers. Linear regression of lance nematode counts and root biomass at each destructive sampling, ANOVA comparing lance nematode counts and nitrogen levels, and ANOVA comparing lance nematode counts and root biomass were analyzed using JMP Pro 18.
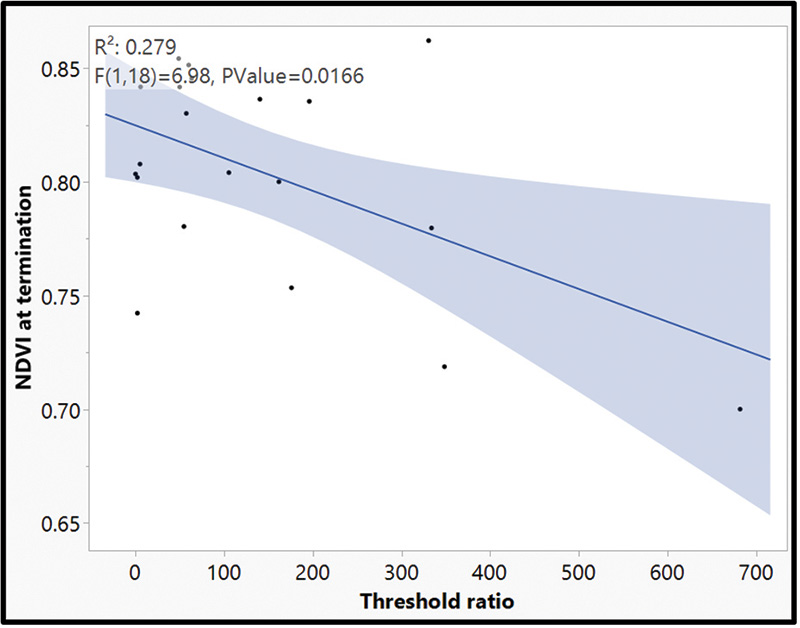
Figure 6. Linear regression of root biomass threshold ratio by normalized difference vegetation index (NDVI) at week 12 termination of creeping bentgrass (Agrostis stolonifera cv. ‘L93’’). Root biomass threshold ratio was determined by evaluating Hoplolaimus galeatus (Cobb, 1913) Thorne, 1935 counts/root biomass (g).
Results and discussion
The results from this research explored new methods of navigating lance nematode identification and quantification by developing alternative action thresholds. The current work adequately addressed both objectives in helping to establish the framework for new alternative action thresholds. First, our data provides clear evidence that lance nematodes can be identified and quantified using a qPCR molecular method. An analysis of variance (ANOVA) of handpicked lance nematodes showed that as nematode counts increased, Ct values decreased (Table 1). When comparing qPCR with random samples, a negative correlation between total H. galeatus counts recorded through elutriation and sugar floatation and Ct values suggest that as counts increase, Ct values decrease (Figure 3). More validation of this work will provide useful, but ultimately there is a clear picture that the methods described herein are successful at identifying and quantifying lance nematodes. These methods will be effective in providing a blueprint for establishing similar methodologies for studying other plant parasitic nematodes that affect turfgrass. Second, our data shows that lance nematodes can have a negative impact on root biomass over time. Destructive samplings at 4, 8 and 16 weeks showed no differences in root biomass by H. galeatus counts, but Week 12 data shows a robust negative correlation that suggests as H. galeatus counts increase, root biomass decreases (Figure 4). Even so, another aspect of this work that must be explored further is the desired effect of nitrogen. The purpose of nitrogen inputs was to provide a gradient of root biomass to provide a sliding action threshold dependent on those inputs. Unfortunately, nitrogen inputs at termination of this study had little to no effect on root biomass (p value = 0.2659); however, the snapshot of data at Week 16 of this greenhouse experiment shows that, over time, elevated lance populations can reduce root biomass (Figure 5). Finally, when assessing plant-health metrics at Week 12, we see a relationship between threshold ratio and Normalized Difference Vegetation Index (NDVI) (Figure 6). This is a snapshot of data that indicates that when lance nematodes are affecting root biomass, they can also be reducing plant health. Overall, working with nematodes is time-consuming, laborious and a bit of a mystery due to the complex nature of how they interact with turfgrass plants. With this work we attempted to address some pressing and challenging issues facing golf course superintendents. This work helped lay a foundation to answer some basic questions regarding alternative action thresholds. Future works will continue to explore the challenges golf course superintendents face regarding plant parasitic nematodes because the best results are often moving the needle on what we know and continuing to explore what we do not.
The research says
- The data provides clear evidence that lance nematodes can be identified and quantified using a qPCR molecular method. More validation of this work will provide useful, but ultimately there is a clear picture that the methods described herein are successful at identifying and quantifying lance nematodes.
- The data shows that lance nematodes can have a negative impact on root biomass over time. Week 12 data show a robust negative correlation that suggests as H. galeatus counts increase, root biomass decreases.
- This work helped lay a foundation to answer some basic questions regarding alternative action thresholds. Future works will continue to explore the challenges golf course superintendents face regarding plant parasitic nematodes.
Acknowledgments
This research was primarily funded by GCSAA and the Virginia GCSA, with supplemental funding from the Virginia Turfgrass Foundation. The authors thank funders for support in advancing knowledge of plant parasitic nematodes in turfgrass systems.
Literature cited
- Bae, C.H., A.L. Szalanski and R.T. Robbins. 2008. Molecular analysis of the lance nematode, Hoplolaimus spp., using the first internal transcribed spacer and the D1-D3 expansion segments of 28S ribosomal DNA. Journal of Nematology 40(3):201 (https://pmc.ncbi.nlm.nih.gov/articles/PMC2664666/).
- Byrd Jr., D.W., K.R. Barker, H. Ferris, C.J. Nusbaum, W.E. Griffin, R.H. Small and C.A. Stone. 1976. Two semi-automatic elutriators for extracting nematodes and certain fungi from soil. Journal of Nematology 8(3):206 (https://pmc.ncbi.nlm.nih.gov/articles/PMC2620186/).
- Crow, W.T. 2021. Managing lance nematodes in warm-season turfgrass. GCMOnline (https://gcmonline.com/course/environment/news/lance-nematodes-turfgrass).
- Settle, D.M., J.D. Fry, T.C. Todd and N.A. Tisserat. 2006. Population dynamics of the lance nematode (Hoplolaimus galeatus) in creeping bentgrass. Plant Disease 90(1):44-50 (https://doi.org/10.1094/PD-90-0044).
- Settle, D.M., J.D. Fry, G.A. Milliken, N.A. Tisserat and T.C. Todd. 2007. Quantifying the effects of lance nematode parasitism in creeping bentgrass. Plant Disease 91(9):1170-1179 (https://doi.org/10.1094/PDIS-91-9-1170).
- Stackhouse, T., A.D. Martinez-Espinoza and M.E. Ali. 2020. Turfgrass disease diagnosis: Past, present, and future. Plants 9(11):1544 (https://doi.org/10.3390/plants9111544).
- Todd, T.C., and N.A. Tisserat. 1990. Occurrence, spatial distribution, and pathogenicity of some phytoparasitic nematodes on creeping bentgrass putting greens in Kansas. Plant Disease 74:660-663 (https://www.apsnet.org/publications/PlantDisease/BackIssues/Documents/1990Abstracts/PD_74_660.htm).
Matthew Aaron Tucker (mat280@vt.edu) is formerly a doctoral student in turfgrass pathology/nematology at Virginia Tech and is currently a new assistant professor of turfgrass management at Auburn University; Jon D. Eisenback is a professor of nematology at Virginia Tech; and David S. McCall is an associate professor of turfgrass pathology and precision turfgrass management at Virginia Tech, Blacksburg.