
Late stages of annual bluegrass weevil damage to golf course fairways in late spring. Photo by Olga Kostromytska
Larvae of the annual bluegrass weevil (ABW, Listronotus maculicollis) cause severe damage to high-profile areas (greens, collars, approaches, tees and fairways) on golf courses in eastern North America. The ABW is the most difficult-to-control insect pest in this region, with two to three generations per year and concurrent presence of different life stages with increasing asynchrony as the growing season progresses (8). Excessive use of synthetic insecticides has led to widespread resistance, often to multiple insecticide classes (6). Newer insecticides have somewhat alleviated control problems, but it is likely that ABW will also become resistant to new chemistries if they are overused.
Entomopathogenic nematodes (EPNs) offer an alternative to synthetic insecticides for ABW management. ABW fourth- and fifth-instar larvae feed from shallow burrows in the thatch or soil at the base of stems, and pupae are found mostly in the top 0.79 inch (2 centimeters) of soil. These stages have been observed to become naturally infected by EPNs and are highly susceptible to the EPN species Steinernema carpocapsae, S. feltiae and Heterorhabditis bacteriophora (4). In spring field trials targeting fourth- and fifth-instar ABW larvae, these species provided 0%-94% control (5). Steinernema carpocapsae provided the most consistent control at rates of 0.5 to 1.0 billion infective juvenile nematodes (IJs)/acre (1.25-2.5 billion IJs/hectare) (60%-69%).
Improvements in EPN efficacy for ABW management may be achieved by splitting treatments into two applications. EPN populations drop quickly after an inundative application in the field (e.g., 5). Because ABW larvae emerge from the grass stems into the soil over a period of several weeks, applications made too early may result in EPN populations declining below effective levels by the time the later-emerging larvae appear in the soil. Absent of a significant decline in EPN efficacy when using only half the rate (5), applying half of the nematodes when fourth instars prevail and the other half around one week later may improve ABW control by EPNs.
EPN infective juveniles under microscope. Photo by Albrecht Koppenhöfer.
Combinations with insecticides could also improve EPN efficacy against ABW larvae. Neonicotinoid insecticides, particularly imidacloprid, have shown mostly synergistic interactions with several EPN species in the control of white grubs (e.g., 1). While imidacloprid only provides limited control of ABW larvae, areas that require ABW management typically also receive preventive insecticide applications for the control of white grubs. Combining applications of EPNs with imidacloprid targeting ABW larvae in late spring could therefore provide both improved ABW control and preventive control of white grubs.
EPNs could be particularly useful against insecticide-resistant ABW populations. Due to the complex nature of the infection process and interaction with the host insect’s immune system, it is unlikely that insects develop resistance to EPN infection if they do not already possess it naturally. However, the mechanism primarily responsible for the insecticide resistance in ABW is enhanced enzymatic detoxification (7). The broad and nonspecific activity of detoxifying enzymes in highly resistant ABW populations could also deactivate some of the compounds produced by the EPNs’ symbiotic bacteria that play important roles in overcoming the insect hosts’ defenses.
The objectives of this study were (a) to determine if splitting EPN applications, combination of EPNs with imidacloprid and the combination of both approaches could improve the control of ABW larvae, and (b) to compare the efficacy of EPNs for the management of ABW populations with different levels of insecticide resistance.
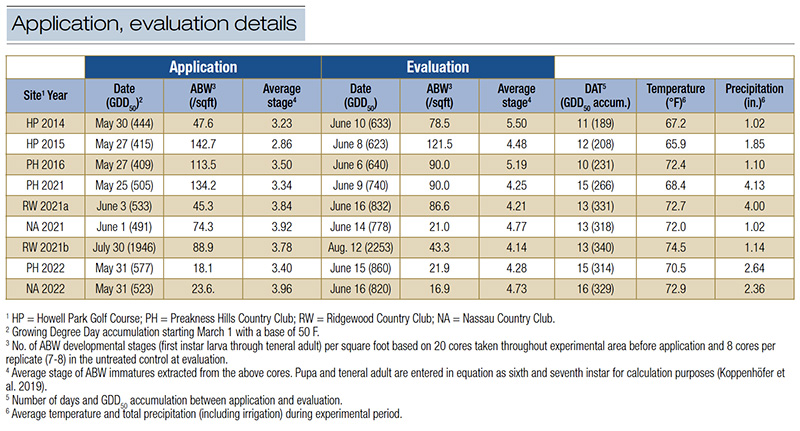
Table 1. Date and Growing Degree Day accumulation, development stage densities and average instar of annual bluegrass weevil (ABW) in untreated control plots at the time of treatment applications, final evaluation, average temperature and total precipitation during experimental period for field experiments testing entomopathogenic nematodes for ABW management.
General methodology
Insects. Four ABW populations with different levels of pyrethroid resistance as determined in previous studies with adults (e.g., 3) were used in this study. Resistance ratios (RR50; ratio of LD50 of tested population to LD50 of the most susceptible population) were based on the population from Rutgers Horticultural Farm II (North Brunswick, N.J.), which had the lowest LD50 values for the pyrethroid bifenthrin in topical assays. Populations were categorized as susceptible (RR50 2.0: HP, Howell Park Golf Course, Manalapan, N.J.); resistant (RR50 55: PH, Preakness Hills Country Club, Wayne, N.J.; RR50 76: RW, Ridgewood Country Club, Paramus, N.J.); and highly resistant (RR50 343: NA, Nassau Country Club, Glen Cove, N.Y.).
EPNs. A commercial strain of the EPN S. carpocapsae (product name: Millennium) was obtained from BASF (Research Triangle Park, N.C.). To standardize quality for experiments, the EPNs were cultured in last instar larvae of the greater wax moth. Imidacloprid (Merit 75 WP) was obtained from Bayer Environmental Science (Montvale, N.J.).
Field experiments. Experiments were arranged in fairway areas with a history of ABW problems along the fairway edge 15 to 19 feet (4.58 to 5.80 meters) deep into the fairway. Individual plots measured 3 feet × 3 feet (0.92 meter × 0.92 meter) with 1-foot (0.31-meter) buffers between adjacent plots. Turf stands at all sites consisted of mixtures of creeping bentgrass (Agrostis stolonifera) and annual bluegrass (Poa annua), mowed at 0.325 to 0.5 inch (8.3 to 12.7 millimeters) height three to four times per week. Experimental areas were managed by golf course staff using the same procedures as the rest of the fairways, except that no insecticides other than the experimental treatments were applied until after experiment evaluation each year.
Time of applications was determined using a combination of growing degree day accumulation (base temperature 50 F (10 C), starting March 1; GDD50), indicator plant phenology and weekly ABW larval sampling. GDD50 was recorded with a WatchDog Weather Tracker Model 300. Flowering of hybrid Catawba rhododendron was observed regularly. Most treatments were applied around the presumed optimal timing for EPNs to infect ABW larvae. This was when a larval instar average of 3.2 was expected (around 480 GDD50, rhododendron still in full bloom), based on previous studies. For split applications, the first half of the treatment was applied at this timing, and the other half about one week later (larval instar average 4.0, around 600 GDD50, late blooming to past blooming of rhododendron). Application dates, GDD50 accumulation, larval densities and instar average for application and evaluation dates are listed in Table 1.
Treatments were applied in 2 gallons per 1,000 square feet (823 liters per hectare) spray volume using a CO2 backpack sprayer at 30 pounds per square inch (207 kPa) pressure with a flat fan nozzle with the screen removed to avoid clogging with the EPNs. Immediately following each application, the experiments received 0.25 inch (6.3 millimeters) of overhead irrigation to wash treatments onto the soil surface. Untreated control plots received only overhead irrigation. In each experiment, treatments were replicated seven to eight times and arranged in a randomized complete block design.
Treatments were evaluated 10-16 days after the last application before new adults were expected to emerge from the soil. To evaluate treatment efficacy, eight cores (2.25 inches/5.7 centimeters diameter × 1.5 inch/4 centimeters depth) were taken from each plot, and ABW stages were extracted by submerging the cores (split into four pieces) for one hour in lukewarm water saturated with table salt. Data were the combined number of larvae (all stages), pupae and teneral adults per plot. Mature adults were not included because, at this time, they were likely to originate from the overwintering generation and might have migrated into the plots from adjacent areas after residual effects of treatments had subsided.
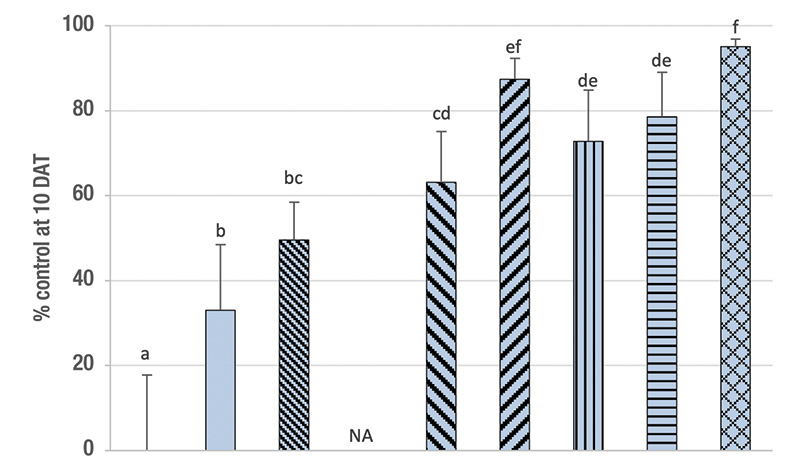
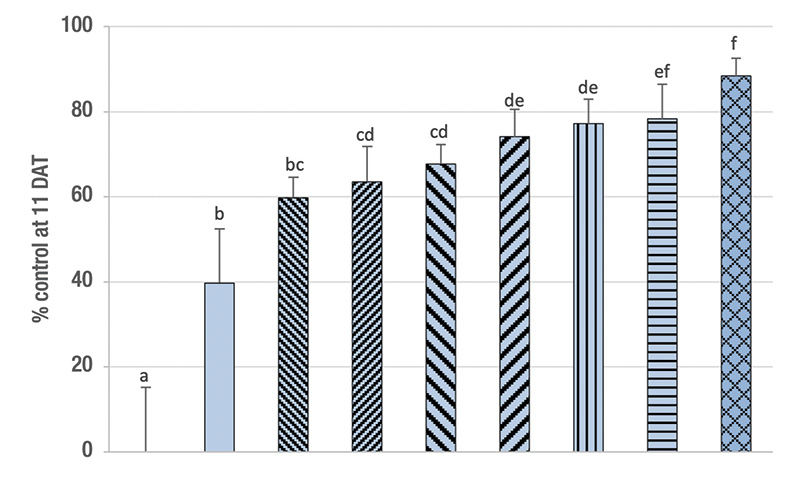
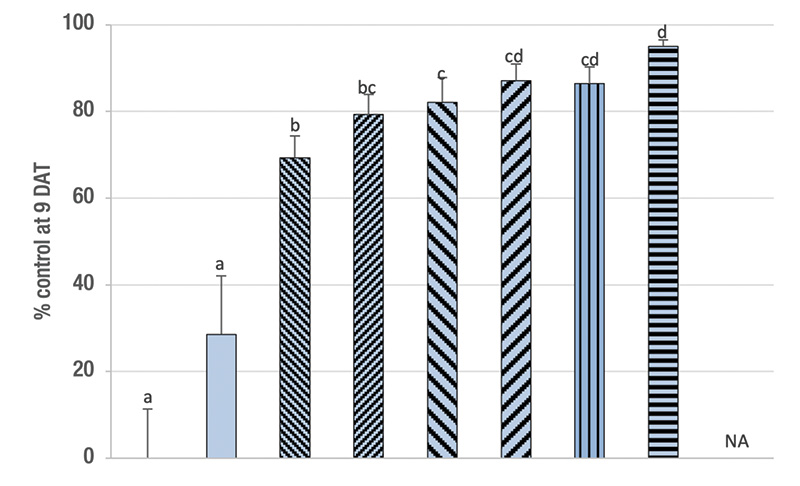
Figure 1. Percentage control (mean ± sem) at 9-11 days after the first treatment (DAT) of ABW developmental stages by single or split (sp) applications of a low and a high rate (0.5/1.0 billion nematodes per acre) of the entomopathogenic nematode Steinernema carpocapsae (C), the insecticide imidacloprid (I; 0.3 pounds active ingredient per acre) and their combinations in naturally infested plots on a golf course fairway in spring. In split applications, half of the nematodes were applied at 0 DAT, the other half at 4-5 DAT. NA = treatments were not applied. Means with the same letter do not differ significantly among treatments (Tukey’s HSD, α = 0.05). Mortality in all combinations was additive (χ2 ≤ 3.84, df = 1, P ≥ 0.05).
Effect of splitting treatments and combinations with imidacloprid
Methods. Three almost identical experiments were conducted in 2014, 2015 and 2016. The full compliment of treatments as used in 2015 was 1) untreated control, 2) imidacloprid (0.3 pounds active ingredient per acre/336 grams active ingredient per hectare), 3) S. carpocapsae at 0.5 billion infective juvenile nematodes (IJs) per acre/1.25 billion IJs per hectare, 4) S. carpocapsae at 1.0 billion IJs per acre/2.5 billion IJs per hectare, 5) split application of low S. carpocapsae rate (two applications of 0.25 billion IJs per acre/0.625 billion IJs per hectare, 6) split application of high S. carpocapsae rate (two applications of 0.5 billion IJs per acre), 7) combination of low S. carpocapsae rate with imidacloprid, 8) combination of high S. carpocapsae rate with imidacloprid, 9) combination of split application of high S. carpocapsae rate with imidacloprid. In 2014, treatment 6 was not included. In 2016, treatment 9 was not included.
Results. Across the three experiments, all treatments provided significant control except for imidacloprid in 2016 (Figure 1). Imidacloprid provided only 28%-39% control. The high S. carpocapsae rate tended to provide higher control (62%-82%) than the low rate (50%-70%), but significantly so only in 2016. The combination treatments always provided additive control that was always significantly higher than the respective single agent treatments with the low S. carpocapsae rate (73%-85%) but not always with the high S. carpocapsae rate (78%-93%). Splitting the S. carpocapsae application tended to increase control over the respective single application, though significantly so only at the high rate and the combination of the high rate with imidacloprid in 2014. The highest level of control was achieved with the split application of the high S. carpocapsae rate combined with imidacloprid (88%-95%).
Effect of insecticide resistance
Methods. An experiment compared the efficacy of S. carpocapsae applied at 0.5 and 1.0 billion IJs per acre/1.25-2.5 billion IJs per hectare and a water-only control against two pyrethroid-resistant (PH, RW) and one highly resistant (NA) ABW population. There were two experiment runs at each site.
Results. Across the three populations and two experiment runs, the high rate of S. carpocapsae provided higher control than the low rate (Figure 2). Control was lower at the highly resistant site (NA) than at one of the resistant sites (RW) but not significantly so than at the other resistant site (PH). At the high rate, S. carpocapsae provided 74% and 67% control of the RW and PH populations, respectively, but only 47% of the NA population.
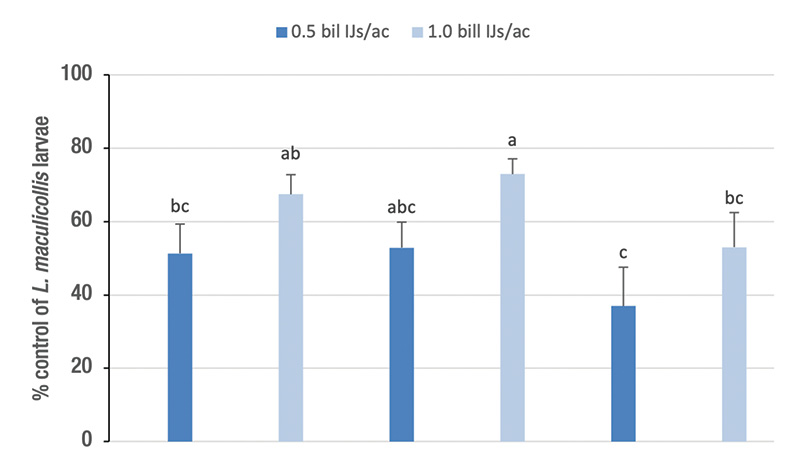
Figure 2. Percentage control (control-corrected; mean ± sem) of ABW developmental stages by the entomopathogenic nematodes Steinernema carpocapsae (0.5 and 1.0 billion nematodes/acre) in naturally infested small plots on golf course fairways with pyrethroid resistant (PH; RR50 for bifenthrin 55; RW; RR50 for bifenthrin 76) and highly pyrethroid resistant (NA; RR50 for bifenthrin 343) ABW populations. Data are combined from two experimental runs in each population (see Table 1). Means with the same lowercase letter do not differ significantly among treatments (Tukey’s HSD, α = 0.05).
Conclusions
Our findings show that a commercial strain of the EPN S. carpocapsae can control ABW larvae on golf course turf. However, the control rate achieved with a single application of S. carpocapsae at the standard rate (1 billion IJs per acre: 62%-82%) would not sufficiently control very high populations of ABW larvae. Splitting the application into two treatments at half rate increased the control by around 10% (74%-87%) into the range typical for synthetic insecticides. Applying the EPNs in two applications rather than one obviously would increase labor. However, golf course superintendents may be able to combine these applications with other routine treatments of herbicides and fungicides and even liquid fertilizer. Many of these agrochemicals are tank-mix compatible with EPNs including S. carpocapsae (2).
Combining the standard rate of S. carpocapsae applied in one treatment with imidacloprid provided excellent control (average: 84%, range 78%-95%) well within the range of synthetic insecticides. The combination would at the same time also control white grubs and would thus not cause an increase in cost and labor. Combining split S. carpocapsae and imidacloprid applications resulted in control as high and consistent (average: 92%; range 88%-95%) as observed for the best synthetic insecticides. We did not test the split low S. carpocapsae rate-imidacloprid combination, but based on our data we would expect a control rate in the range of 80%-85%, which would be still as good as most synthetic insecticides, while making the combination more economical.
EPNs would be particularly useful alternatives for insecticide-resistant ABW populations. Unfortunately, S. carpocapsae efficacy was somewhat reduced against the highly resistant population. However, against more moderately resistant populations, the efficacy was not affected. And the S. carpocapsae-imidacloprid combination was also similarly effective against susceptible and moderately resistant ABW populations. It may be worthwhile to explore potential interactions of EPNs with newer insecticides such as spinosyn (Provaunt) and cyantraniliprole (Ference) that to date do not seem to be affected by resistance. Moreover, the vast majority of ABW populations should either not be affected by resistance or have levels of resistance at which EPN efficacy should not be reduced. Hence, EPNs generally remain a useful tool in the management of ABW and in the delay of insecticide resistance development.
The research says
- The EPN S. carpocapsae can provide good control of ABW larvae, albeit not suffiently high and consistent to always control very high ABW populations.
- Splitting treatments into two applications applied 5-7 days apart improves S. carpocapsae performance by around 10% and also reduces control variability.
- Combining S. carpocapsae with imidacloprid increases S. carpocapsae performance by around 10% and also reduces control variability.
- Split applications of S. carpocapsae combined with imidacloprid provide control comparable with the most effective synthetic insecticides.
- S. carpocapsae efficacy is reduced against highly insecticide-resistant ABW populations, but both S. carpocapsae alone and its combination with imidacloprid should be highly effective against the vast majority of ABW populations that are not highly insecticide resistant.
Funding
This research was supported by grants from the Rutgers Center for Turfgrass Science, USGA, O.J. Noer Research Foundation, New York State Turfgrass Association, Tri-State Turf Research Foundation and the USDA National Institute of Food and Agriculture Hatch Multistate project 0206130 through the New Jersey Agricultural Experiment Station (Hatch Multistate project NJ08295).
Acknowledgments
The authors thank the maintenance staff of Howell Park Golf Course, Preakness Hill Country Club, Ridgewood Country Club and Nassau Country Club for help with the field study. This article was based on two published papers, “Optimizing the use of entomopathogenic nematodes for the management of Listronotus maculicollis (Coleoptera: Curculionidae): Split applications and combinations with imidacloprid” by A.M. Koppenhöfer, O.S. Kostromytska and S. Wu in 2020 in Crop Protection (137: 2022.105229); and “Effect of pyrethroid resistance on the efficacy of entomopathogenic nematodes for the control of Listronotus maculicollis (Coleoptera: Curculionidae)” by A.M. Koppenhöfer, A.L. Sousa, O.S. Kostromytska and S. Wu in 2025 in Biological Control (200: 2024.105683).
Literature cited
- Koppenhöfer, A.M., and E.M. Fuzy. 2008. Early timing and new combinations to increase the efficacy of neonicotinoid-entomopathogenic nematode (Rhabditida: Heterorhabditidae) combinations against white grubs (Coleoptera: Scarabaeidae). Pest Management Science 64:725-735 (https://doi.org/10.1002/ps.1550).
- Koppenhöfer, A.M., and S. Foye. 2024. Interactions with agrochemicals and biological control agents. Pages 494-518. In: D.I. Shapiro-Ilan, E.E. Lewis, eds. Entomopathogenic nematodes as biological control agents. CABI Publ., Wallingford, UK (https://doi.org/10.1079/9781800620322.0027).
- Kostromytska, O.S., S. Wu and A.M. Koppenhöfer. 2018. Cross-resistance patterns to insecticides of several chemical classes among Listronotus maculicollis (Coleoptera: Curculionidae) populations with different levels of resistance to pyrethroids. Journal of Economic Entomology 111:391-398 (https://doi.org/10.1093/jee/tox345).
- McGraw, B.A., and A.M. Koppenhöfer. 2009. Population dynamics and interactions between endemic entomopathogenic nematodes and annual bluegrass weevil populations in golf course turfgrass. Applied Soil Ecology 41:77-89 (https://doi.org/10.1016/j.apsoil.2008.09.002).
- McGraw, B.A., R. Cowles, P.J. Vittum and A.M. Koppenhöfer. 2010. Field evaluation of entomopathogenic nematodes for the biological control of the annual bluegrass weevil, Listronotus maculicollis (Coleoptera: Curculionidae) in golf course turfgrass. Biocontrol Science & Technology 20:149-163 (https://doi.org/10.1080/09583150903440658).
- McGraw, B.A., and A.M. Koppenhöfer. 2017. A survey of regional trends in annual bluegrass weevil (Coleoptera: Curculionidae) management on golf courses in eastern North America. Journal of Integrated Pest Management 8:1-11 (https://doi.org/10.1093/jipm/pmw014).
- Ramoutar, D., R.S. Cowles and S.R. Alm. 2009. Pyrethroid resistance mediated by enzyme detoxification in Listronotus maculicollis Kirby (Coleoptera: Curculionidae) from Connecticut. Journal of Economic Entomology 102:1203-1208 (https://doi.org/10.1603/029.102.0345).
- Vittum, P.J. 2020. Turfgrass insects of the United States and Canada, third edition. Cornell University Press, Ithaca, N.Y.
Albrecht M. Koppenhöfer (a.koppenhofer@rutgers.edu) is an Extension specialist in the Department of Entomology, Rutgers University, New Brunswick, N.J. Olga S. Kostromytska is an Extension assistant professor in the Stockbridge School of Agriculture, University of Massachusetts, Amherst. Shaohui Wu is an assistant professor in the Department of Entomology, Ohio State University, Columbus. Ana Luiza Sousa is a research scientist at Syngenta Seeds Development-R&D Innovation Center, Malta, Ill.