
Figure 1. Monitoring insect activity involves looking for clues to identify the culprits. This grass has suffered from the presence of billbugs, which feed on turfgrass crowns. Photo by Dave Shetlar
One of the most critical steps in creating an integrated pest management (IPM) plan is to monitor pest activity throughout the growing season. This article describes several techniques that can be used to monitor insect activity. Unlike monitoring pathogen activity, scouting for insects is often fairly straightforward, because the insects are usually big enough to see using a variety of techniques.
Scouting for turf insects: Use the clues around you
When you see turf damage, you should think of yourself as a crime scene investigator and look for clues that might help narrow your search for the cause. The first piece of evidence involves looking at the damage (Figure 1). Does it appear to be caused
by insects or pathogens or something abiotic? This article will focus on insect damage, but symptoms can be confusing. For example, annual bluegrass weevil damage can look very much like anthracnose damage in June if you look only at the superficial
damage and fail to notice larvae feeding in the thatch.
What does damage look like?
Many aspects of turf damage can provide clues as to which insect species might be involved. Are there several discrete patches, each of a similar size and appearance, or is the damage more variable (Figures 2 and
3, below)? Is the turf intact, or is there evidence of feeding? If there is feeding, does it occur on the roots or stems or blades? Can the affected turf be pulled up easily? What color are the patches?

Figure 2. Although the scars on the fairway may bear little resemblance to the nearly barren sections of the rough, annual bluegrass weevil was the guilty party in both cases. Photo by Ben McGraw

Figure 3. This turf suffered extreme damage caused by the presence of insects, but not by the insects themselves. Skunks severely injured the turf as they foraged for a feast of grubs. Photo by Pat Vittum
Where does damage occur?
Does the damage occur on all the grasses, or does it occur only on certain species or cultivars? Which playing surfaces are affected? Damage caused by the same insect often looks very different on greens and surrounds
than it does on tees, fairways or roughs. Are there certain conditions or locations that seem to coincide with damage? Examples include edges of fairways, south-facing slopes, sandy soils, heavy soils, soils with poor drainage and areas near sprinkler
heads.
When does damage occur?
Most turf damage that is caused by insects occurs about the same time each growing season. Growing degree day accumulation and plant phenology (timing of certain plant developmental stages, such as full bloom) can
often be helpful in predicting which insects might be present at a given time. Indeed, many universities base their management recommendations for turf and ornamental plants on growing degree day accumulations instead of calendar dates. Make notes
when you notice damage, and record any trees or shrubs that are just reaching full bloom or any other notable stage of development. As you add notes each year, your field observations will help you recognize what might be happening with seasonal pest
activity.
What environmental conditions are present at the time of damage?
Many insects will thrive in certain conditions and do less well in others. For example, soil moisture is important. Some insects, such as invasive crane flies, survive better
when soils are almost saturated at the time of egg-laying or larval emergence. Japanese beetles and some other scarabs like soil that is moderately moist, while European chafer grubs prefer drier conditions. Some insects feed only on one or two species
of grass. For example, the annual bluegrass weevil prefers annual bluegrass in the northern part of its range, but it will also feed on creeping bentgrass in the southern parts of its range. Many insects develop better in areas with thick or dense
thatch.
Make notes on the agronomic conditions in the areas where you observe insect damage, including soil texture, soil moisture, soil temperature, thatch thickness or density, effectiveness of drainage, and uniformity of irrigation coverage. And, of course,
note the species or cultivars that are affected, as well as the mowing heights that are most vulnerable to the damage.
Active turf insect scouting techniques

Figure 4. Often, the search for insects is as basic as getting down on your hands and knees and teasing apart the grass, as illustrated by this researcher’s hunt for chinch bugs. Photo by Dave Shetlar
Many simple techniques can be used to scout for turf insect activity throughout the golf course (Table 1).
Some techniques involve actively looking for insects — and getting a sense of how many insects occur in a given area (often a square foot) (Figure 4). The effectiveness of each technique depends on having a good idea of where the insects are most
likely to be. It is important to understand the life cycles of the most common turf insects that are likely to occur in your area, so you know what time of year to be looking for them. And it is important to know which kinds of conditions those insects
prefer, so you can concentrate your scouting efforts in the most likely areas. You are not likely to find very many black cutworms on a Kentucky bluegrass rough, for example, as black cutworms do not grow well on Kentucky bluegrass, but you may notice
activity on the neighboring creeping bentgrass green.
The techniques described below can be used to scout for insect activity on golf course turf. Additional techniques have been developed for monitoring insects in trees and shrubs.
Click on table to enlarge and download:
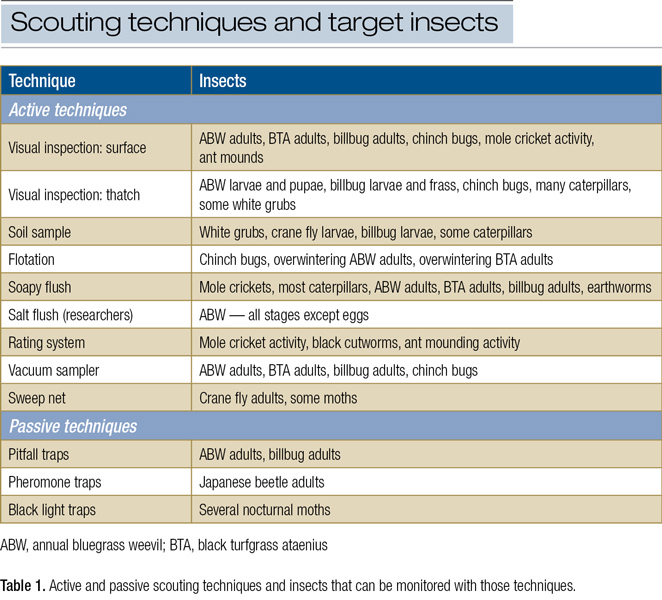
Visual inspection
The simplest way to scout for turf insects is to get down on your hands and knees and look for them! You might kneel and tease apart the upper layers of the thatch, looking for insects or movement or telltale
deposits. For example, annual bluegrass weevils can be seen moving on the surface even though they are quite small (Figure 5, below). Billbug larvae produce frass (insect fecal deposits) in the thatch that looks very much like sawdust (Figure
6, below). Visual inspection can also be conducted on a sample of turf that has been cut out using a knife or cup-cutter. Tease the thatch apart and look for insects that are active in the thatch, including annual bluegrass weevil larvae, billbug
larvae and several species of caterpillars.

Figure 5. Even small insects like annual bluegrass weevils (shown here) and chinch bugs can be spotted and identified as they move on the turf’s surface. Photo by Charlie Kerr

Figure 6. Recognizing an insect’s distinctive fecal matter (or “frass”) is another way to determine which pest is responsible for turfgrass injury. The billbug frass in this photo bears a strong resemblance to sawdust. Photo by Dave Shetlar
Visual inspection is useful in scouting for annual bluegrass weevil adults and larvae, black turfgrass ataenius adults, chinch bugs, billbug adults and larvae, billbug frass, mole cricket tunnels, ant mounds, and some caterpillars.
Soil sample
The best way to scout for insects that feed on turf roots or deep in the thatch (such as white grubs, annual bluegrass weevil larvae or billbug larvae) is to take a sample that includes the foliage, thatch and roots.
You can use a cup-cutter or a small garden spade (4 to 6 inches; 10 to 15 centimeters) to cut a square to a depth of 2 to 4 inches (5 to 10 centimeters). A cup-cutter has an area very close to 0.1 square foot (93 square centimeters), which makes
determining the number of insects per square foot easy.

Figure 7. Using a trowel to knock off the soil from the underside of a turf sample will allow you to see grubs and other insects that are feasting on the grass. Photo by Olga Kostromytska

Figure 8. Grubs are light in color and highly visible in soil. Photo by Dave Shetlar
Use the edge of a hand trowel to gently pound and dislodge the soil from the underside of the sample (Figure 7). Any grubs that are in the sample will be visible against the dark soil. Place the grubs in a muffin tin, and count the number of grubs
per plug. Note that soil samples are often not effective for monitoring mole cricket activity, because mole crickets can move faster than we can dig. Although cream-colored white grubs are easily seen in soil, crane fly larvae tend to be brown
or dark green, so they are not quite as obvious (Figure 8).
A soil sample can be used to scout for annual white grubs, Phyllophaga grubs, black turfgrass ataenius grubs, crane fly larvae, billbug larvae (found in soil and thatch) and some caterpillars.
Flotation
To collect a flotation sample, remove both ends of an empty coffee can and insert it (or a similar-sized cylinder) through the thatch into the soil, and fill the can with water (Figure 9, below). An alternative approach
is to take a turf sample with a cup-cutter, cut the plug into three or four smaller pieces, put the plugs in a bucket, and fill the bucket with water. The goal is to submerge the sample in water so that most insects must either float to the surface
or drown. Insects in a sample will usually come to the surface fairly quickly (within a minute or two).

Figure 9. A coffee can makes an ideal container for collecting flotation samples. Photo by Pat Vittum
Flotation can also be used to sample leaf litter in nearby wooded areas. Just collect about a square foot of leaf litter, place it in a plastic dishpan, and fill the pan with lukewarm water. Place white paper towels on the surface and wait for insects
to float to the top. Most of them will cling to the underside of the paper towel, making it much easier to see them. It might take some insects as long as 20 to 30 minutes to make their way to the surface of the leaf litter.
Flotation is useful when scouting for chinch bug adults and nymphs, annual bluegrass weevil adults (especially from leaf litter in overwintering sites), and black turfgrass ataenius adults (especially from overwintering sites).
Soapy flush/disclosing solution
Many entomologists use a “soapy flush” (sometimes called a disclosing solution) to irritate insects in the turf and induce them to move to the surface. Produce a soapy mixture by adding 1 to 2 tablespoons
(~15 to 30 milliliters) of lemon-scented dish detergent to a gallon of water. Use a watering can to apply about a pint (~0.5 liter) of the mixture to an area that is about 1 square foot (929 square centimeters), and apply another pint about five
minutes later. Sensitive insects will begin to climb to the surface within a couple of minutes — and will often climb to the tips of grass blades, trying to get away from the irritation (Figure 10, below). Check for insects before the second application,
and again five, 10 and 15 minutes after the second application of soapy water. It is important to use lemon-scented detergent, because it is very irritating to many kinds of insects.

Figure 10. This annual bluegrass weevil was brought to the surface of the grass after a soapy flush (disclosing solution) was applied. Photo by Ben McGraw
Note that soapy flushes produce a soapy foam on the surface that can act almost like a magnifying glass and can burn the turf, so do not conduct a soapy flush in the middle of a hot summer day in full sunlight. In addition, you may want to apply plain
water to the area after you have finished counting insects, to dilute the soap and start to move it out of the sunlight.
A soapy flush can be used to scout for mole crickets, many caterpillars, annual bluegrass weevil adults, black turfgrass ataenius adults, billbug adults and earthworms. Some small caterpillars may die before they reach the surface, so you may underestimate
their population.
Salt flush
Salt flushes are sometimes used by researchers to extract small weevil or billbug larvae from stems and thatch. The approach is based on the concept that salty water is an irritant and also makes everything more buoyant.
Researchers prepare a saturated salt solution by dissolving about 1 cup of table salt in a quart of lukewarm water and pouring it into beakers that contain turf samples cut into small (1-square-inch; 6.45-square-centimeter) pieces, using enough
water to completely cover the samples. Then, stir the solution every 10 minutes for about an hour and record all the insects that pop to the surface. Larvae (including tiny larvae that are inside the stem), pupae and adults of annual bluegrass
weevils are extracted using this technique. A small magnifying glass is often needed to see the smallest larvae, but they often wiggle and create a small, clear space in the briny water, making them easier to spot.
One of the challenges that makes using this technique difficult for superintendents is that you may generate large amounts of salty water that are difficult to dispose of. But the technique does enable you to determine when the tiniest larvae are
hatching and thereby time your insecticide applications more accurately.
A salt flush is used for research on population development of annual bluegrass weevils, and it can also work on billbugs.
Rating system
About 30 years ago, Pat Cobb, Ph.D. (now retired from Auburn University), developed a rating system to provide a way for superintendents to quantify mole cricket activity. She built a frame 2 feet (61 centimeters)
on a side using 1-inch-diameter (2.5-centimeter-diameter) PVC pipe, and attached two eye bolts on each side of the frame equidistant from the end. She tied string from each eye bolt to the one opposite it on the frame, creating a frame with nine
equally sized squares (Figure 11, below).

Figure 11. Pat Cobb, Ph.D., devised a simple grid to check for mole cricket activity. Her system can also be used to monitor ant mounds or cutworm feeding damage. Photo by Pat Vittum
Cobb would toss the frame randomly in the area she was sampling and conduct several counts at each location by inspecting each small square in the frame for evidence of mole cricket activity (burrowing or tunneling). If a square showed activity, it
was scored as “1”; if no activity was evident, it was scored as “0.” After all the squares had been inspected, she would total the scores. The total would range from 0 (no activity in any square) to 9 (activity in every square). This simple device
enables a superintendent to track the severity of mole cricket activity seasonally and compare activity from one year to another.
The rating system is used to document mole cricket activity and could also be used to count ant mounds or assess black cutworm feeding damage.
Vacuum
A relatively new technique involves using a leaf blower/vacuum shredder, adjusted to act as a vacuum, to collect samples from the surface of the turf. Insert a collecting bag or net in the open end of the vacuum cylinder
to prevent any insects or detritus from being sucked into the motor, and drag the cylinder over the area to be sampled (Figure 12, below). The collecting bag should be sturdy enough to withstand the vacuum flow of the machine, but should allow
enough air to pass through to create the vacuum. Several kinds of butterfly nets work well.

Figure 12. Leaf blowers and vacuum shredders can be modified and turned into vacuum samplers to collect insects such as annual bluegrass weevil adults, billbug adults, black turfgrass ataenius adults and chinch bugs. Photo by Pat Vittum
It is wise to choose a length of time (for example, 45 seconds or one minute) to run the machine in the sample area so you can compare numbers from one sample to another. You also want to be as consistent as possible in sampling at the same time of
day and under similar weather conditions. Insects are often more active on the surface on sunny days than on cloudy or rainy days.
A vacuum sampler can be used to scout for annual bluegrass weevil adults, billbug adults, black turfgrass ataenius adults and chinch bugs.
Sweep nets
Sweep nets are fine-meshed insect netting on a hoop attached to a long-handled pole and can be used to collect some flying insects. Use a figure-eight motion in front of your body to move the net through the air quickly,
catching any insects that are flying in the area. When you are done, encircle the net with your hand near the top of the net and gradually move your hand down toward the far end of the net, slowly forcing the insects to the end. Be aware that
you may have collected some stinging insects inadvertently. Quickly place an empty jar in the gap, shake the insects into the jar, and then slip a lid onto the jar. You may be able to identify the insects immediately, or you can put the jar in
a refrigerator or freezer for a few minutes to slow insect activity.
Sweep nets are very good tools to scout for crane fly adults and various moths.
Passive turf insect scouting techniques (traps)
The techniques described above involve doing something to find the insects, while other approaches call for trapping insects in some fashion. Some traps attract insects to them, and others involve capturing them as they move through an area.
Pitfall traps
Pitfall traps are devices designed to trap insects that fall into the device. Traps can be purchased, or you can make them yourself. Some traps are constructed using plastic cups (cut a hole with a cup-cutter and
insert the cup so the lip is level with the surface, backfilling around the cup if necessary). Linear pitfall traps (Figure 13, below) for annual bluegrass weevils or billbugs are constructed by cutting a slit on each end of a 2- to 3-foot
(0.6- to 0.9-meter) length of PVC pipe. Each of the two slits should be about 0.25 inch (0.64 centimeter) wide and slightly less than half the length of the pipe.

Right: Figure 13. Linear pitfall traps, which can be purchased or built from simple materials, are ideal passive scouting devices for capturing annual bluegrass weevil adults or billbugs. Photo by Dave Shetlar
Insert the pipe in the ground with a slight incline and with the slits on the upper surface, so the top of the pipe is level with the surface. Cut a hole in the side of a collecting device — for example, a 1- or 2-liter (33.8- or 67.6-ounce) plastic
soda bottle — that matches the diameter of the PVC pipe, and insert the collecting device at the low end of the tube. Place a cap on the top of the bottle. As insects walk along the surface, they will fall through the slit into the tube and eventually
make their way to the low end of the tube, where they will accumulate in the collecting bottle.
Traps can be checked regularly (once or twice a week) and can indicate when a population is increasing rapidly. If you only check the trap once a week, you may want to use some kind of preservative in the collecting device to prevent insects from
escaping. Rubbing alcohol can work, although it will be diluted if rain fills the bottle, and it may evaporate if the bottle is not covered. Antifreeze is an excellent preservative but is extremely toxic to dogs and cats, so it is not recommended.
Pitfall traps are excellent for tracking spring adult activity of annual bluegrass adults, billbug adults and black turfgrass ataenius adults.
Pheromone traps
Pheromones are chemicals produced by animal species, including insects, to communicate with others of the same species. There are many different kinds of pheromones that perform different functions. One common
pheromone is a “sex pheromone,” produced by a female to indicate her presence to males of the same species. Chemists have been able to identify the molecular structures of several insect sex pheromones, and these are used as lures in traps. One
of the most familiar of such traps is a Japanese beetle trap, which contains a sex pheromone to attract males and a floral scent to attract both sexes. These traps are good at attracting large numbers of beetles to the vicinity of the trap and
are excellent for monitoring adult activity (Figure 14, below). However, they do not reduce the population, and in many cases, damage from Japanese beetles is more severe near traps than in similar areas where traps are not deployed.

Figure 14. Sex pheromones are used as lures to attract Japanese beetles to traps. Pheromone traps are highly effective for monitoring Japanese beetles, but they do not reduce (and may increase) the insect’s population size in areas where they are installed. Photo by Ben McGraw
The sex pheromone for the oriental beetle has been identified, and research (by Albrecht Koppenhöfer, Ph.D., at Rutgers University) documented that mating can be disrupted if the pheromone is distributed across a large area. So far, however, that
pheromone has not been commercialized.
Pheromone traps are effective for monitoring Japanese beetle activity. Other pheromones have been identified but are not available commercially for trapping.
Black light traps
Many nocturnal insects are attracted to light during their nighttime flight. Black light traps draw insects toward the trap after dark. As the insects hit the vertical baffles, they draw in their legs to drop
to the ground but instead fall into a collecting device placed below the light source. These traps can attract large numbers of insects, and identifying all the individuals can be challenging, but they can certainly give an indication of when
insect activity is on the increase.
Black light traps can be used to monitor moths of several of the turf-damaging species, as well as annual bluegrass weevil adults in summer and adults of some white grub species, such as May beetles and European chafers.
Conclusions
Monitoring insect activity is critical in determining when (and whether) insects are active and likely to cause damage on the golf course. The techniques described here will give you a good chance of tracking that activity and planning management
strategies based on what you find.
Acknowledgment
Special thanks to Laurie Brocklesby for her careful reading and editing of this manuscript and all the others I have written over the past 20 years.
The research says ...
- The first steps in monitoring insect activity are to determine what the damage looks like, where and when it occurs, and what environmental conditions are present at the time of damage.
- In active scouting, you can look for the insects, take soil or flotation samples, or use a soapy flush or salt flush to bring insects to the surface.
- A rating system is a tool for documenting activity of mole crickets, ants and black cutworms, and vacuums and sweep nets can be used to collect insects.
- Passive scouting involves pitfall, pheromone and black light traps that capture insects and help superintendents determine population levels.
References
- Brandenburg, Rick L., and Callie P. Freeman. 2012. Handbook of turfgrass insects. Entomological Society of America, Lanham, Md.
- Koppenhöfer A.M., S. Polavarapu, E.M. Fuzy, A. Zhang, K. Ketner and T. Larsen. 2006. Mating disruption of oriental beetle with sprayable sex pheromone formulations. USGA Turfgrass and Environmental Research Online 5(15):1-8 (https://usgatero.msu.edu/v05/n15.pdf).
- Potter, Daniel A. 1998. Destructive turfgrass insects: Biology, diagnosis, and control. Sleeping Bear Press, Ann Arbor Press, Chelsea, Mich.
- Vittum, Patricia J. 2020. Turfgrass insects of the United States and Canada, 3rd ed. Comstock Publishing, Cornell University Press, Ithaca, N.Y.
Pat Vittum is professor emeritus at the University of Massachusetts-Amherst specializing in turfgrass entomology, and is also affiliated with the university’s Center for Agriculture, Food and the Environment. In 2017, she became the first woman to be honored with the GCSAA Col. John Morley Distinguished Service Award.