
Figure 1. Stand symptoms characteristic of take-all root rot on ultradwarf bermudagrass. Note the white patches that range from 6 to 24 inches in diameter. Photos by Cameron Stephens
Take-all root rot (TARR) is a prevalent and destructive disease of ultradwarf bermudagrass (Cynodon dactylon x Cynodon transvaalensis; UDB) putting greens caused by multiple ectotrophic root-infecting fungi (Gaeumannomyces graminis [Gg], Gaeumannomyces
graminicola [Ggram], Gaeumannomyces sp. [species unknown; Gx], Candidacolonium cynodontis [Cc] and Magnaporthiopsis cynodontis [Mc]) (2, 8, 9, 12, 13). TARR has been the most frequently diagnosed disease of UDB samples submitted to the Turf Diagnostics
Lab at North Carolina State University since 2016 and remains challenging for turfgrass managers (1). On UDB, foliar symptoms of TARR appear as white-to-off-color patches ranging from 4 to 48 inches in diameter (Figure 1). Under periods of increased
plant stress, patches may coalesce to form larger nondistinct patches of thinning turf. Older turfgrass plant leaves appear chlorotic, and crown necrosis can be observed.
This disease appreciably affects aesthetics and playability of UDB wherever it is used for golf course putting green turf (2, 4, 13). Fungicide applications targeting TARR are most commonly applied in the fall once symptoms are present. This curative
approach has not been an effective method for TARR control, as golf course superintendents are often left with symptoms on their putting greens until the UDB can grow out of the symptoms the following spring.
Fungicide applications are typically required to effectively manage TARR. However, data on fungicide efficacy and application timing on TARR management under field conditions is limited. The dense, perennial nature of established turfgrass systems and
presence of organic matter at the soil surface (thatch) create a unique challenge for managing TARR. TARR is elusive under field conditions, and finding reliable research sites with uniform disease development is difficult. Since TARR symptoms have
yet to be reproduced at a research farm, multiple off-site trial locations are required, and disease intensity at each site varies.
Stephens et al. (10) determined fungicides from the demethylase inhibitor (DMI) and strobilurin (QoI) chemical classes can be effective at reducing TARR pathogen growth in vitro, whereas fungicides from the succinate dehydrogenase inhibitor (SDHI) chemical
class did not reduce pathogen growth. Research by Martin (5) aligns with this conclusion by demonstrating QoI and DMI fungicides suppressed TARR symptoms, while SDHI fungicides provide limited disease suppression under field conditions. Field research
conducted on St. Augustinegrass (Stenotaphrum secundatum) managed for home lawn turf revealed applications of azoxystrobin and triadimefon reduced TARR severity by 30% and 35%, respectively (6).
Similarly, research on UDB showed two applications of tebuconazole, triadimefon + trifloxystrobin, or fluxapyroxad + pyraclostrobin can reduce TARR severity to below 10% when TARR severity was >50% in the nontreated control plots and the SDHI fluxapyroxad
did not reduce TARR severity compared to the non-treated control (5). Similarly, fungicide applications in mid-August as opposed to mid-July to early-August resulted in a greater reduction of TARR symptoms that developed in December and January. The
mid-August applications resulted in a 60% reduction in TARR severity compared to the non-treated control, suggesting applying efficacious fungicides in late summer may optimize fungicide efficacy. While soil temperatures at the time of application
were not recorded in the aforementioned studies, Stephens et al. (11) conducted in vitro temperature studies with Gg, Gx, Ggram, Cc and Mc isolated from symptomatic UDB to determine their growth temperature optima. Each pathogen grew optimally between
77 F and 86 F (25-30 C) and exhibited limited growth at 95 F (35 C) and no growth at 50 F (10 C). Correlating growth temperature optima with soil temperature at a specific location may be an informative strategy to trigger fungicide applications for
TARR when the pathogens are most actively growing.
While field trials are paramount for understanding practical TARR management, greenhouse assays using inoculated UDB may be a useful tool for investigating fungicide efficacy. Therefore, the objectives of this research were to improve our understanding
of fungicide application timing and fungicide efficacy using field and in vivo studies. These data will provide important information to turfgrass managers on how to better manage this detrimental disease.

Table 1. Influence of trifloxystrobin + triadimefon application initiation timing on take-all root rot severity on an ultradwarf bermudagrass putting green at Walnut Creek Country Club and Mill Creek Golf Course.
Materials and methods
Fungicide application timing studies 1 and 2
Two, two-year field trials were conducted in 2019 and 2020 in Alamance, Durham and Wayne counties in North Carolina to evaluate the influence of fungicide application timing on TARR management. The field trial sites were composed of Champion UDB built
to USGA specification and maintained at a height of 0.1 inch (2.54 millimeters). The first timing study conducted at Mill Creek Golf Club (Timing Study 1) included treatments of mefentrifluconazole (Maxtima, BASF Corporation, Research Triangle Park,
N.C.) and mefentrifluconazole + pyraclostrobin (Navicon, BASF Corporation, Research Triangle Park, N.C.) applied on a calendar-based schedule on Aug. 27 and Sept. 17 or Oct. 28 and Nov. 19. Average daily soil temperatures at these locations for 2019
and 2020 at a 2-inch (5-centimeter) depth were 81, 73, 62 and 51 F (27, 22.8, 16.7 and 10.5 C) on Aug. 27, Sept. 17, Oct. 28 and Nov. 19, respectively (7).
The second timing study conducted at Walnut Creek Country Club (Timing Study 2) evaluated the influence of trifloxystrobin + triadimefon (Tartan, Bayer Crop Science, Cary, N.C.) applied four times on a 21-day interval with variable initiation dates of
July 20, Aug. 10, Aug. 31, Sept. 21, Oct. 9, Oct. 29 and Nov. 19 (Table 1). The average daily soil temperature at a 2-inch depth was 87 F (30.5 C) on July 20, 80 F (26.7 C) on Aug. 10, 77 F on Aug. 31, 78 F (25.5 C) on Sept. 21, 64 F (17.8 C) on Oct.
9, 62 F on Oct. 29 and 50 F on Nov. 19 (7).
Fungicides for all field studies were applied using a CO2-powered pressurized sprayer at 50 pounds per square inch coupled to an air-induction flat fan nozzle (AI9508EVS, TeeJet Spraying Systems Company, Glendale Heights, Ill.) calibrated to deliver 2
gallons per 1,000 square feet (815 liters per hectare). Immediately after fungicide application, plots received 0.25 inch (0.64 centimeter) of post-application irrigation using the overhead irrigation system. Disease severity was assessed visually
every 21 days after application by estimating the percent of turf within each plot exhibiting symptoms characteristic of TARR. Sites were regularly visited throughout the winter and spring to monitor for symptom development.

Figure 2. Plants were maintained in a greenhouse set to 85 F/75 F (29 C/24 C) day/night temperature for seven days until fungicides were applied. They received 0.125 inch (0.32 centimeter) of overhead mist irrigation daily and were hand-trimmed to a height of 0.25 inch every other day.
Fungicide efficacy in the greenhouse
Colonized rye grain (Secale cereale) was used to inoculate UDB to evaluate the efficacy of fungicides on TARR under greenhouse conditions. Bermudagrass plugs (1.0-inch [2.54-centimeter] diameter) were harvested from a healthy, newly sprigged Champion
putting green located at the Turfgrass Field Laboratory in Raleigh, N.C. The root system was then removed, and the remaining stolons, rhizomes and above-ground plant material were vigorously washed with water. Next, 5 cubic centimeters of rye grain
infested with Gaeumannomyces graminis and Gaeumannomyces graminicola was added to respective conetainers (1.5-inch diameter by 8.25-inch depth/3.8 by 20.95 centimeters), and a bermudagrass plug was placed directly on top of the inoculum and topdressed
with 5 cc sand. Plants were maintained in a greenhouse set to 85 F/75 F (29 C/24 C) day/night temperature for seven days until fungicides were applied. They received 0.125 inch (0.32 centimeter) of overhead mist irrigation daily and were hand-trimmed
to a height of 0.25 inch every other day (Figure 2).
Respective fungicide treatments can be found in Table 2 and were applied using the field fungicide application method previously described. Two fungicide applications were made 21 days apart starting seven days after inoculation and disease severity in
the containerized system was visually assessed using a Horsfall-Barratt scale (3).
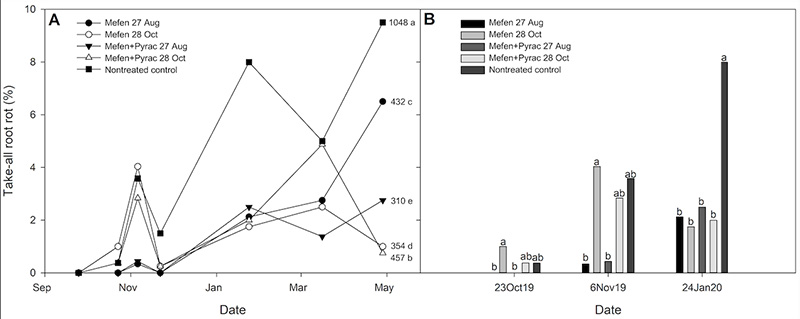
Figure 3. Influence of Maxtima (mefentrifluconazole; Mefen; 0.9 pounds active ingredient per acre) and Navicon (mefentrifluconazole + pyraclostrobin Mefen+Pyrac; 0.5 pounds active ingredient per acre) application timing on take-all root rot severity at a golf course in Chapel Hill in 2019. Maxtima (Mefen Aug. 27) and Navicon (Mefen+Pyrac Aug. 27) were applied on Aug. 27, 2019, and Sept. 17, 2019. Maxtima (Mefen Oct. 28) and Navicon (Mefen+Pyrac Oct. 28) were applied on Oct. 28, 2019, and Nov. 19, 2019. A: Represents disease progression over time with numbers after each treatment representing the area under disease progress curve (AUDPC) values. B: Represents dates on which there was a significant treatment effect. Mean AUDPC values in panel A and mean take-all root rot severity (%) within each rating date in panel B followed by the same letter are not statistically different according to Fisher’s Protected least significant difference test (α=0.05).
Results
Timing study 1.
In 2019, TARR was first observed on Oct. 23 and steadily increased throughout the winter. Disease severity peaked in the non-treated control plots April 28, 2020 (Figure 3A). All treatments resulted in significantly lower area under disease progress curve
(AUDPC = disease over time) values compared to the non-treated control (1048) (Figure 3A). Applications of Maxtima (Mefen) and Navicon (Mefen + Pyrac) on Aug. 27 reduced TARR severity that developed in November when compared to the later treatments
and non-treated control (Figure 3A). All treatments reduced TARR severity on the Jan. 24, 2020, rating date, yet symptoms never totally dissipated in this trial in any treatment (Figure 3A).
In 2020, TARR was first observed on Aug. 27, 2020, remained consistent until Sept. 17, then rapidly increased and peaked at 15.2% in the non-treated control plots on Nov. 19 (Figure 4A). Based on AUDPC values, all treatments except for Maxtima (Mefen
Oct. 28) reduced TARR severity compared to the non-treated control (Figure 4A). The best treatment in 2020 was when Maxtima and Navicon (Mefen and Mefen + Pyrac) were applied on Aug. 27 (Figure 4A and 4B).
Timing study 2.
TARR in the trifloxystrobin + triadimefon application timing trials progressed differently in 2019 and 2020. In 2019, TARR activity was only observed on Jan. 6, 2020, and was numerically the highest in the non-treated control plots at 9.7% (Table 1).
Trifloxystrobin + triadimefon applications beginning on Aug. 7, 2019, (Timing 2) was the only treatment that reduced TARR severity (1.7%) (Table 1)
In 2020, TARR was first observed on Aug. 27 and increased throughout the duration of the trial, peaking at 17.3% in the non-treated control on Nov. 19 (Table 1). Application timings did not influence TARR severity until Oct. 29. From Oct. 29 throughout
the remainder of this trial, Timing 1, 2 and 3 significantly reduced TARR severity when compared to the non-treated control. AUDPC values were statistically lower with Timing 1, 2 and 3 treatments compared to the non-treated control, and Timing 2
was significantly better than Timing 1 (Table 1).
In vivo fungicide efficacy
On Sept. 15, Sept. 28 and Oct. 12, all treatments reduced TARR severity compared to the non-treated control. Pots treated with azoxystrobin, mefentrifluconazole + pyraclostrobin, and azoxystrobin + difenoconazole were among the lowest statistical grouping
for disease severity on Sept. 15 and Oct. 12. On Sept. 28, plants treated with benzovindiflupyr alone and benzovindiflupyr + difenoconazole had higher disease severity than other fungicide treated pots (Table 2).

Figure 4. Influence of Maxtima (mefentrifluconazole Mefen; 0.9 pounds active ingredient per acre) and Navicon (mefentrifluconazole + pyraclostrobin Mefen+Pyrac; 0.5 pounds active ingredient per acre) application timing on take-all root rot severity at Mill Creek Golf Course in 2020. Maxtima (Mefen Aug. 27) and Navicon (Mefen+Pyrac Aug. 27) were applied Aug. 27, 2020, and Sept. 17, 2020. Maxtima (Mefen Oct. 28) and Navicon (Mefen+Pyrac Oct. 28) were applied on Oct. 28, 2020, and Nov. 19, 2020. A: Represents disease progression over time with numbers after each treatment representing the area under disease progress curve (AUDPC) values. B: Represents dates on which there was a significant treatment effect. Mean AUDPC values in panel A and mean take-all root rot severity (%) within each rating date in panel B followed by the same letter are not statistically different according to Fisher’s Protected least significant difference test (α=0.05)
Conclusions
Root diseases such as TARR are challenging to manage and even more challenging to conduct management research on. Inducing disease is not as simple as one might think, so having a method to conduct studies in the greenhouse is a valuable tool for researchers
to screen many fungicides for this disease. Although field studies are of paramount importance, this work demonstrates that greenhous studies can be a suitable alternative and provide corroborative support to field trials. Based on the data collected
from this study, it is clear that fungicides within QoI and/or DMI classes should be the backbone of any TARR management program. However, timing is also critical, as is proper post-application irrigation practices. All the treatments in this study
received at least 0.125 inch of post-application irrigation immediately after applications. In some cases, 0.25 inch was applied, so golf course superintendents should be cognizant of not only active ingredient but also the post-application irrigation
strategy as well. With regard to timing, the best treatments in these studies were initiated when soil temperatures were between 80 and 86 F. From this work, we suggest initiating applications when soil temperatures drop to 86 F in late summer or
early fall and continue every 21 days until soil temperatures drop below 68 F (20 C).
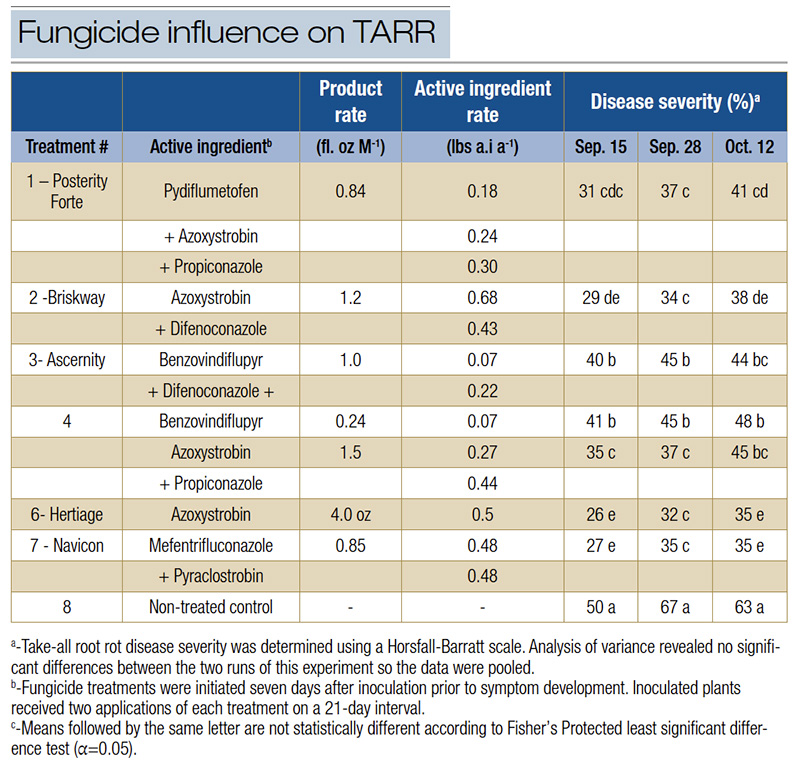
Table 2. Influence of preventive fungicide applications on take-all root rot disease severity following in vivo inoculation with Gaeumannomyces graminis and G. graminicola.
The research says
- Fungicides from the QoI and/or DMI chemical class provided the greatest reduction in take-all root rot severity.
- Apply an efficacious fungicide when the 5-day average soil temperature at a 2-inch depth is approximately 80 F for TARR management.
- The in vivo screening method developed here is an efficient way to evaluate TARR management parameters.
Literature cited
- Butler, L. 2020. Turf pathology turf files diagnostic lab review. North Carolina State University.
- Elliott. M.L. 1991. Determination of an etiological agent of bermudagrass decline. Phytopathology 81:1380-1384.
- Horsfall, J.G., and R.W. Barratt. 1945. An improved grading system for measuring plant disease. Phytopathology 35:655.
- Landschoot, P.J., and N. Jackson. 1990. Pathogenicity of some ectotrophic fungi and Phialophora anamorphs that infect the roots of turfgrasses. Phytopathology 80:520-526.
- Martin, S.B. 2017. Take-all root rot on the increase in the Carolinas. Golf Course Management (https://gcmonline.com/course/environment/news/take-all-root-rot-on-the-increase-in-the-carolinas).
- Martinez-Espinoza, A., D. Gardner and J. Price. 2005. Management of Gaeumannomyces graminis (take-all root rot) on St. Augustinegrass in coastal Georgia using fungicides and soil amendments. Phytopathology 95.
- North Carolina State Climate Office. 2023. Cardinal: Station Scout. https://products.climate.ncsu.edu/cardinal/scout/.
- Stephens, C.M. 2021. Etiology, epidemiology, and management of take-all root rot on golf course putting greens. Ph.D. dissertation. North Carolina State University.
- Stephens, C.M., and J.P. Kerns. 2020. First report of Gaeumannomyces graminicola causing bermudagrass decline of ultradwarf bermudagrass putting greens in North Carolina. Plant Disease 104:1552.
- Stephens, C.M, T.W. Gannon, L.D. Thiessen, M.A. Cubeta and J.P Kerns. 2023. In vitro fungicide sensitivity and effect of organic matter concentration on fungicide bioavailability in take-all root rot pathogens isolated from North Carolina. Plant Health
Progress 24:2 (https://doi.org/10.1094/PHP-08-22-0072-RS).
- Stephens, C.M., T.W. Gannon, M.A. Cubeta, T.L. Sit and J.P. Kerns. 2022. Characterization and aggressiveness of take-all root rot pathogens isolated from symptomatic bermudagrass putting greens. Phytopathology 112:4.
- Vines, P.L. 2015. Evaluation of ultradwarf bermudagrass cultural management practices and identification, characterization and pathogenicity of ectotrophic root-infecting fungi associated with summer decline of ultradwarf bermudagrass putting greens.
Master of Science thesis. Mississippi State University.
- Vines, P.L., F.G. Hoffman, F. Meyer, T.W. Allen, J. Luo, N. Zhang and M. Tomaso-Peterson. 2020. Magnaporthiopsis cynodontis, a novel turfgrass pathogen with widespread distribution in the United States. Mycologia 122:52-63 (https://doi.org/10.1080/00275514.2019.1676614).
Cameron M. Stephens (cstephens.472@gmail.com) is a product development manager and fungicide lead at ADAMA; Marc A. Cubeta is a professor and associate director of the Center for Integrated Fungal Research, and James P. Kerns is a professor, Extension specialist of turfgrass pathology and University Faculty Scholar, both in the Department of Entomology and Plant Pathology, North Carolina State University; and Travis W. Gannon is a professor of pesticide fate and behavior in the Department of Crop and Soil Science, North Carolina State University.