
A low-maintenance experiment was set up at the Lake Wheeler Turfgrass Field Laboratory at North Carolina State University in October 2018 to evaluate six cultivars of tall fescue. Photo by Jialin Hu
Drought poses a major challenge to agricultural production, negatively impacting crop physiology and reducing soil nutrient availability. Tall fescue (Festuca arundinacea) is generally considered a low-maintenance grass with deep and extensive root systems
that can adapt to and survive short drought periods. For this reason, it is a popular turfgrass to use on golf courses in the rough or out-of-play areas. With a desire to increase water conservation, breeding new cultivars with greater stress tolerance
has become more important. Studies have shown that soil and phyto microorganisms are able to improve plant drought resistance by regulating phytohormone production, scavenging reactive oxygen species (ROS) and improving plant nutrient acquisition
(3). However, the extent and manner by which microbes confer plant drought resistance are plant species- and microhabitat-specific (8), and the relevant knowledge is still lacking for turfgrass.
Microbial community composition varies among the root endosphere, rhizosphere and adjacent bulk soil (4). Such microhabitat differentiation often comes with different microbial roles in sustaining plant growth in a water-stressed environment. For instance,
root endophytic microbes are likely to be more involved in regulating phytohormones and antioxidants (9), while mycorrhizal fungi are heavily involved in transporting water and nutrients to host plants (5). Microhabitat differentiation in the rhizosphere
and root endosphere microbiomes varies with plant species and genotypes and even at the cultivar level, as observed on economic crops (e.g., barley, maize and potato). This raises a fundamental yet practically important question: Should phyto-microbiomes
be taken into consideration in the plant breeding program for developing varieties with better environmental adaptability? For example, the gram-positive, oligotrophic bacteria, such as Actinobacteria, Chloroflexi and Firmicutes, are often found to
be abundant under drought because their thicker cell walls and smaller cell sizes make them more resistant to desiccation and easier to survive in low-moisture and nutrient-poor conditions (3). If their abundance is proven to be associated with the
drought-tolerance behavior of cultivars, these bacteria may be used as the phenotype to help reliably screen stress-tolerant cultivars.
In this study, we aimed to evaluate tall fescue cultivars for drought-adaptive ability from the perspective of belowground microbial communities, considering that plant-associated microbiota is the “extended phenotype” of the host. We also
attempted to propose elite microbes in helping confer tall fescue drought resistance by linking microbial taxa with the cultivar quality rating under drought.
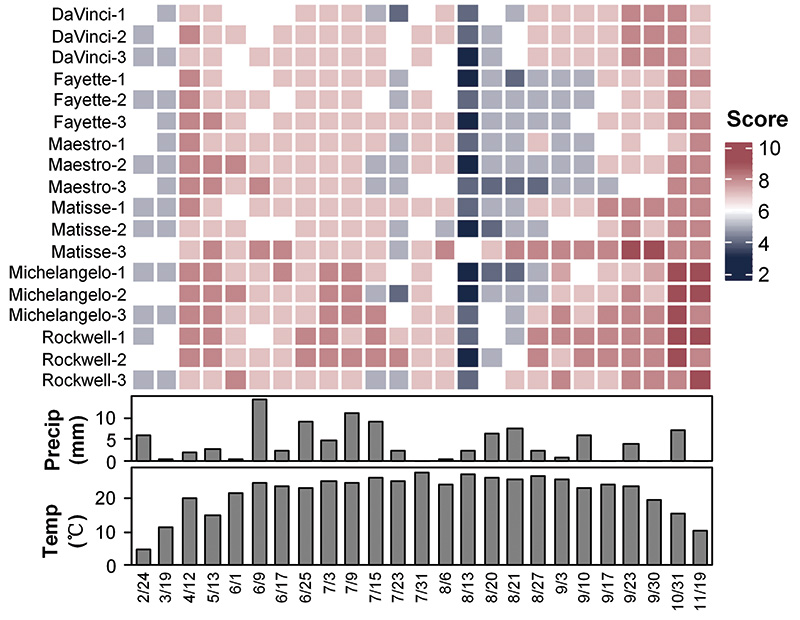
Figure 1. Heatmap of the plant visual quality rated on a scale from 1 to 9, with 1 being a brown canopy and 9 being a dark green and uniform canopy for six tall fescue cultivars under no irrigation during the year 2021 at irregular intervals. Bar plots represent the averaged precipitations and temperatures for seven days before each quality rating day.
Materials and methods
A low-maintenance experiment was set up at the Lake Wheeler Turfgrass Field Laboratory at North Carolina State University in October 2018 to evaluate how six cultivars of tall fescue (DaVinci, Fayette, Maestro, Matisse, Michelangelo and Rockwell) would
perform under no irrigation, i.e., precipitation as the sole source of water for plant growth. Tall fescue cultivars were randomly arranged into a total of 18 4.5-foot-by-4.5-foot (1.37-meter-by-1.37-meter) plots in a randomized complete block design
with three blocks. All plots were professionally managed for fertilization and herbicide applications in the same manner. The quality of tall fescue cultivars under no irrigation was assessed by visual ratings during the year 2021 at irregular intervals.
In late September 2021, intact grass-soil cores were taken from each plot, leading to a total of 18 samples. In addition, four intact grass-soil cores were collected as irrigated controls from the adjacent irrigated area of a multicultivar blend of
tall fescue (Rain Dance, Coronado and Cumberland), where overhead irrigation was conducted every other day at approximately 0.20 to 0.25 inches (0.51 to 0.64 centimeters) of water per irrigation.
The grass roots, rhizosphere soil and bulk soil were separated from the grass-soil cores in the laboratory for genomic DNA extraction. Quantitative polymerase chain reaction (qPCR) was performed with the DNA extracted from each sample to quantify total
bacterial and fungal abundances. The soil moisture, pH and inorganic nitrogen concentrations (ammonium and nitrate) were measured for bulk soil samples. High-throughput amplicon sequencing of bacterial 16S rRNA gene and fungal ITS regions combined
with bioinformatics software DADA2 and QIIME2 were used to reveal the bacterial and fungal community composition and diversity in the roots, rhizosphere and bulk soil. The abundances of bacterial functional genes related to phytohormones, antioxidant
enzymes and nutrient acquisition were predicted based on bacterial community results using PICRUSt2. For normally distributed data, one-way analysis of variance with post hoc least significant difference test was used to evaluate the effects of microhabitats,
cultivar and irrigation on soil physicochemical properties, microbial abundances and alpha diversity indices. For non-normally distributed data, the difference between irrigation and no irrigation was tested by the Mann-Whitney U test, and the difference
among cultivars was tested by the Kruskal-Wallis test with Dunn’s post hoc test. Statistically significant difference level at P<0.05 was used in this study unless otherwise noted.
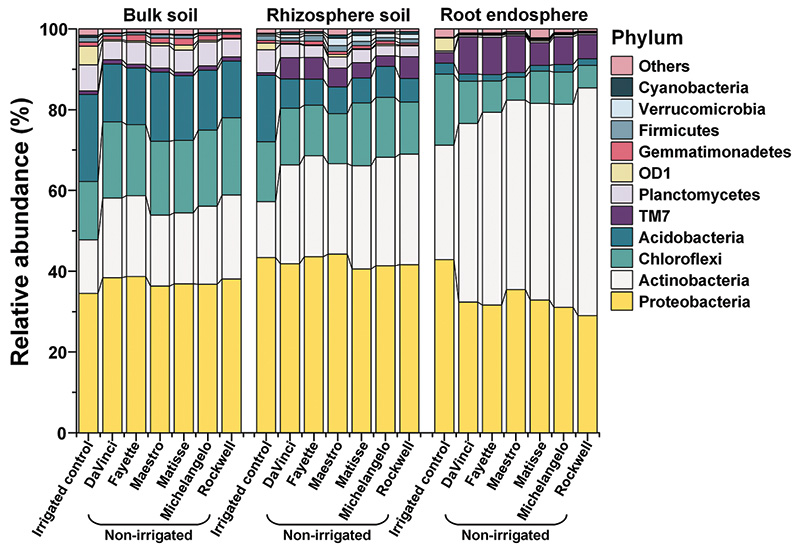
Figure 2. Relative abundance of the bacteria at the phylum level as affected by microhabitats, irrigations and cultivars.
Results and discussion
Tall fescue, a cool-season perennial C3 bunch-type turfgrass, grows vigorously in spring and fall but may go dormant in hot and dry summer days or wintertime when temperatures drop below 50 F (10 C). Indeed, the quality of all the six tall fescue cultivars
under no irrigation had gone down moderately in late winter/early spring and severely in the middle of August (Figure 1). We were not surprised by the grass turning brown under no irrigation because of the low precipitation and high temperature from
the end of July to the middle of August. Measured in early October, soil moisture under no irrigation was only ~10%, threefold lower than the soil moisture under irrigation (~40%), suggesting that grasses were under water stress. Obviously, cultivars
(or genotypes) differed in resilience from the summer dormancy, with Rockwell performing best and Fayette and Maestro performing worst (Figure 1).
The microbial community composition was significantly impacted by microhabitat and water stress and varied among cultivars. In general, the bacterial community was dominated by the phyla Proteobacteria and Actinobacteria (Figure 2). Proteobacteria in
the rhizosphere and bulk soil were affected little by irrigation treatments, averaging ~43% in the rhizosphere and ~36% in bulk soil, but in the root endosphere were significantly reduced by no irrigation, i.e., ~32% under no-irrigation compared to
~43% under irrigation. Actinobacteria were enriched by no irrigation in all three microhabitats and peaked at ~49% in the root endosphere. In detail, no irrigation led to ~1.8-, 1.8- and 1.5-fold increases in relative abundance of Actinobacteria in
the root endosphere, rhizosphere and bulk soil, respectively, compared to irrigation. Enrichment in the relative abundance by no irrigation was also manifested in most sublevel taxa of Actinobacteria in the root endosphere and rhizosphere, but less
in bulk soil. Further, enrichment by no irrigation in the relative abundance of phylum Actinobacteria and its major sublevel taxa, including Actinobacteria (class), Actinomycetales (order), Streptomycetaceae (family), and Streptomyces (genus) in the
root endosphere varied significantly with cultivars, which were more abundant in Rockwell, Michelangelo and Matisse. Such a compositional shift suggested that Actinobacteria were the key bacteria for coping with the water stress of tall fescue.
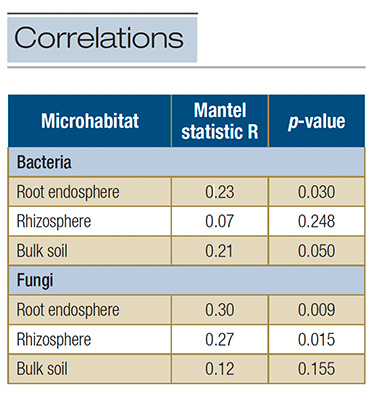
Table 1. Correlations between the plant quality ratings and the distance matrix of microbial communities in the root endosphere, rhizosphere and bulk soil based on the Mantel test.
In fact, there were significant positive associations between the relative abundances of endophytic Actinobacteria and its dominant members, including the putative abundance of bacterial functional genes related to phytohormone production and nutrient
acquisition (data not shown). For example, the genus Streptomyces was positively correlated with genes that encode enzymes catalyzing phytohormone production, such as ACC (1-aminocyclopropane-1-carboxylate) deaminase and auxins (Spearman correlation
coefficient R>0.75 and p<0.01 for both). By sequestering and cleaving plant-produced ACC, the immediate precursor of phytohormone ethylene, ACC deaminase can help reduce the level of ethylene in the plant and therefore promote plant drought
tolerance (1). Moreover, root endophytic bacteria might also help promote plant drought adaptation through nutrient acquisition, given that several abundant endophytic Actinobacterial taxa, such as Streptomyces reticuliscabiei, Micromonosporaceae
and Sphaerisporangium, were positively associated with bacterial functional genes involved in nitrogen transformations (e.g., nitrate reduction to ammonium, ammonium assimilation and extracellular nitrogen mineralization) and phosphate solubilization
(R>0.50 and p<0.01 for both). This widespread phenomenon implies that species of the phylum Actinobacteria are likely elite bacteria for improving plant drought tolerance and may be used as the phenotypes of plant genotypes for quick and reliable
screening of drought-tolerant cultivars in the plant breeding program.
If the tall fescue-growth-promoting endophytic microbes solely belonged to Actinobacteria under drought, the cultivar DaVinci would be expected to have the worst performance, because its roots contained the lowest abundance of Actinobacteria. However,
DaVinci was found to be healthier than Fayette and Maestro under drought, suggesting that other microbes also contributed to the drought resistance of tall fescue. In this study, we observed that DaVinci and Rockwell were more pronounced in promoting
the relative abundance of fungal phylum Basidiomycota in the root endosphere and of Mortierellomycota and Basidiomycota in the rhizosphere soil as well. DaVinci was also better in promoting the arbuscular mycorrhizal fungi Glomeromycota and its dominant
sub-level taxa in bulk soil (Figure 3). Actually, DaVinci was comparable to Rockwell, the best drought-tolerant cultivar, in terms of the relative abundance of Basidiomycota and Glomeromycota. Taking advantage of penetrating pores inaccessible to
roots and redistributing water from moist to dry areas, Glomeromycota help alleviate drought on plants by improving the supply of water and nutrients to their host plants (2, 5, 6). Basidiomycota likely confer the tolerance of tall fescue against
drought via osmolyte regulation, ROS scavenging or water retention in the host (7). Thus, we considered that Basidiomycota and Glomeromycota were the elite fungi for improving tall fescue drought tolerance.
Our results illustrated the importance of root-associated microbes in screening drought-tolerant tall fescue cultivars. We found that visual quality ratings of the cultivars were fairly well related to changes in microbial community compositions in the
root endosphere and/or rhizosphere (Table 1). Unlike microbial community composition, the soil physicochemical properties as well as the bacterial and fungal abundance and diversity (i.e., species richness and evenness) were generally not significantly
different among the six cultivars under water stress.
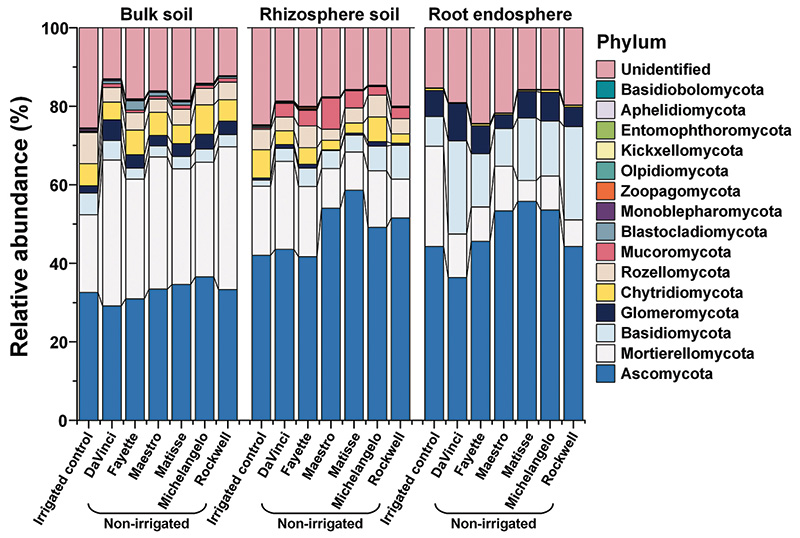
Figure 3. Relative abundance of the fungi at the phylum level as affected by microhabitats, irrigations and cultivars.
Conclusions
This work uses different cultivars of tall fescue to address a basic question in the plant breeding program: whether the root-associated microbes can be viewed as the phenotypes of plant genotype for helping screen stress-tolerant crop cultivars. A few
bacterial and fungal taxa in the roots and the rhizosphere soil, including Actinobacteria, Basidiomycota and Glomeromycota, were found to positively respond to water stress, yet magnitude varied among cultivars, suggesting their suitability for appraising
cultivar-specific stress fitness. This inference was also supported by concerted change between the relative abundance of drought-responsive microbial taxa and the visual quality score of tall fescue cultivars. The tight connection between drought-responsive
Streptomyces and putative genes encoding for phytohormone regulation underscores the possible mechanics of microbes for conferring tall fescue with water stress tolerance. Drought responses are obvious from the phylum to genus level for Actinobacteria,
but mainly at the phylum level for mycorrhizal and endophytic fungi. In addition, drought responses of Actinobacteria appear widespread, while drought responses of Basidiomycota may not be observed in all turfgrasses. More work is needed for understanding
how ecological factors interfere with the development of the root-associated Basidiomycota and Glomeromycota under drought.
Funding
This research was funded by the North Carolina Turfgrass Center for Environmental Research and Education. This research article is intended for educational purposes. Further comprehensive information can be accessed in our original publication in Frontiers
in Microbiology (https://doi.org/10.3389/fmicb.2022.1078836).
The research says
- Cultivars of tall fescue differed in resilience from the summer dormancy, with Rockwell performing best and Fayette and Maestro performing worst.
- Microbial community composition in the bulk soil, rhizosphere soil and endosphere of tall fescue was significantly affected by water deficit.
- Tall fescue cultivars slightly, yet significantly, modified root endophytic microbial communities under water stress.
- Cultivars showing better adaptability to drought encompassed more relatively abundant Actinobacteria, Basidiomycota or Glomeromycota in roots and the rhizosphere, compared to poorly performing cultivars.
- Root endophytic microbes have high potential to be used for screening drought-adaptive cultivars of turfgrass.
Literature cited
- El-Tarabily, K.A. 2008. Promotion of tomato (Lycopersicon esculentum Mill.) plant growth by rhizosphere competent 1-aminocyclopropane-1-carboxylic acid deaminase-producing streptomycete actinomycetes. Plant and Soil 308(1-2):161-174 (https://link.springer.com/article/10.1007/s11104-008-9616-2).
- Ignacio Querejeta, J., L.M. Egerton-Warburton, I. Prieto, R. Vargas and M.F. Allen. 2012. Changes in soil hyphal abundance and viability can alter the patterns of hydraulic redistribution by plant roots. Plant and Soil 355(1/2):63-73 (https://link.springer.com/article/10.1007/s11104-011-1080-8).
- Naylor, D., and D. Coleman-Derr. 2017. Drought stress and root-associated bacterial communities. Frontiers in Plant Science 8:2223 (https://doi.org/10.3389/fpls.2017.02223).
- Naylor, D., S. DeGraaf, E. Purdom and D. Coleman-Derr. 2017. Drought and host selection influence bacterial community dynamics in the grass root microbiome. The ISME Journal 11(12):2691-2704 (https://www.nature.com/articles/ismej2017118).
- Parniske, M. 2008. Arbuscular mycorrhiza: the mother of plant root endosymbioses. Nature Reviews Microbiology 6(10):763-75 (https://www.nature.com/articles/nrmicro1987).
- Poudel, M., R. Mendes, L.A.S. Costa, C.G. Bueno, Y. Meng, S.Y. Folimonova, et al. 2021. The role of plant-associated bacteria, fungi, and viruses in drought stress mitigation. Frontiers in Microbiology 12:743512 (https://doi.org/10.3389/fmicb.2021.743512).
- Verma, A., N. Shameem, H.S. Jatav, E. Sathyanarayana, J.A. Parray, P. Poczai and R.Z. Sayyed. 2022. Fungal endophytes to combat biotic and abiotic stresses for climate-smart and sustainable agriculture. Frontiers in Plant Science 13:953836 (https://doi.org/10.3389/fpls.2022.953836).
- Wang, P., E.L. Marsh, G. Kruger, A. Lorenz and D.P. Schachtman. 2020. Belowground microbial communities respond to water deficit and are shaped by decades of maize hybrid breeding. Environmental Microbiology 22(3):889-904 (https://pubmed.ncbi.nlm.nih.gov/31163094).
- Xu, L., A. Wang, J. Wang, Q. Wei and W. Zhang. 2017. Piriformospora indica confers drought tolerance on Zea mays L. through enhancement of antioxidant activity and expression of drought-related genes. The Crop Journal 5(3):251-8 (https://doi.org/10.1016/j.cj.2016.10.002).
Jialin Hu, Ph.D., (jhu36@ncsu.edu) is a postdoctoral research scholar; Grady Miller, Ph.D., is a professor, Extension Turfgrass Specialist and ENVU Distinguished Professor of Sustainability; and Wei Shi, Ph.D., is a professor of soil microbiology and ecology, all in the Crop and Soil Sciences Department at North Carolina State University, Raleigh.